Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 3
5-year survival and prognostic factors for resectable colon cancer: A single institution experience in Lebanon
Rhea Mattar*; Fadi El Karak; Marwan Ghosn
Department of Hematology-Oncology, Hotel-Dieu de France University Hospital, Saint Joseph University, Beirut, Lebanon.
*Corresponding Author : Rhea Mattar
Department of Hematology-Oncology, Hotel-Dieu
de France University Hospital, Saint Joseph
University, Beirut, Lebanon.
Tel: +96 171464840;
Email: rheamattar28@gmail.com
Received : Jun 18, 2023
Accepted : Jul 07, 2023
Published : Jul 14, 2023
Archived : www.jjgastro.com
Copyright : © Mattar R (2023).
Abstract
Background: This study aims to analyze the 5-year Overall Survival (OS) and the Progression-Free Survival (PFS) of patients with localized CC as well as studying the impact of various prognostic factors. It’s the first study evaluating survival and prognostic factors for resectable CC in the region.
Patients and methods: Medical records of 79 patients at Hotel-Dieu de France (HDF) hospital were reviewed. OS and PFS were analyzed using Kaplan-Meier method.
Results: Advanced stages, advanced grades, and vascular invasion at the time of colectomy were correlated with a lower 5-year OS and PFS, with a statistically significant association. No significant association was observed between cancer sidedness and survival after colectomy.
Conclusion: Survival and recurrence after CC resection remain important problems. Tumor stage, tumor grade, and vascular invasion are prognostic factors that affect survival after colectomy.
Keywords: Colon cancer; Colectomy; Survival analysis; Overall survival; Progression-free survival; Recurrence.
Citation: Mattar R, Karak FE, Ghosna M. 5-year survival and prognostic factors for resectable colon cancer: A single institution experience in Lebanon. J Gastroenterol Res Pract. 2023; 3(5): 1147.
Introduction
Colon Cancer (CC) is, by its frequency and severity, a major public health problem. It’s one of the leading causes of cancer morbidity and mortality worldwide. It’s the third most frequently diagnosed cancer in men and the second in women. It’s the third leading cause of cancer death in both men and women [1].
The survival of CC depends on the stage of the disease at the time of diagnosis and the response to treatment. The 5-year survival rate decreases as the stage increases: it is greater than 90% for stage I and less than 15% for stage IV. Early detection would therefore be important and effective [2].
About 90% of patients with CC are treated surgically [3]. Radical surgical resection is the standard treatment for AJCC stage I to III CC. Adjuvant Chemotherapy (CT) is given to patients with high-risk stage II and stage III [4]. High-risk patients are those with the following characteristics: Stage T4, perforation or obstruction, low grade, lymphatic and vascular invasion, less than 12 nodes examined and a high preoperative Carcinoembryonic Antigen (CEA) [5].
Although most patients diagnosed at localized stages (stages I, II and III) recover, 35% develop a recurrence, mainly within the first 5 years [6].
Published rates of survival and recurrence after colectomy vary widely [7]. In the Middle East, and more specifically in Lebanon, there are very few studies on this subject.
Lebanon ranks second in terms of incidence of CC among countries in the Middle East and North Africa (MENA) region with increasing incidence over the past few years [8]. Data collection is not easy in Lebanon and the National Cancer Registry (NCR) was inactive for many years due to unstable political and economic situation. Indeed, several data are missing in the Lebanese NCR such as overall survival (OS) and progression-free survival (PFS) after surgical resection of CC.
Our objective is to study the OS at 5 years and the PFS of patients with CC at localized stages after surgical resection in our institution, and this by analyzing the impact of several prognostic factors such as tumor stage, sidedness, grade, size, patient's age at the time of colectomy and adjuvant CT.
Materials and methods
Study estimates and sampling
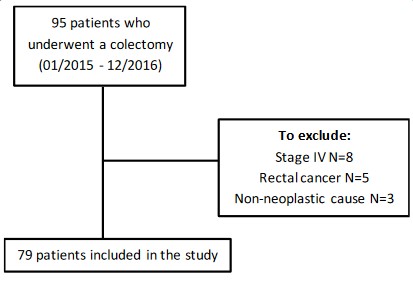
This is an applied survival study in patients who underwent surgical resection for CC at localized stage between January 2015 and December 2016 at Hotel-Dieu de France (HDF) hospital (Beirut, Lebanon). The medical records of 95 patients who underwent primary CC resection were reviewed. The information was collected from the medical records of the patients, present in the archives of the hospital and in the clinics of the attending physicians. The patients were followed and included in a database until January 2022 or until their death if this occurred before January 2022.
Primary tumors located at the level of the cecum, ascending colon and transverse colon were defined as tumors of the right colon, while those located at the level of the splenic angle, descending colon and sigmoid colon were defined as tumors of the left colon. The AJCC TNM staging system (8th edition) was used for staging.
OS was defined as the time from resection of the primary tumor to death from any cause. PFS was defined as the time from the date of surgery to the date of detection of recurrence, last follow-up, or death.
We excluded patients who underwent surgical resection for stage IV CC, and for a non-neoplastic cause as well as those who had rectal cancer. The final sample included 79 patients who underwent curative resection for stage I, II or III CC (Figure 1).
Ethical considerations
The protocol and all the study procedures were approved by the ethics committee of Saint-Joseph University in Beirut. The Helsinki declarations of 1963 were considered: respect, confidentiality, and patient anonymity.
Statistical analysis
Data were analyzed using SPSS software version 29. The categorized variables were compared by Pearson’s χ² test, and quantitative variables were compared by the Student’s t-test. OS and PFS were analyzed using the Kaplan–Meier method. P values less than 0.05 were considered statistically significant.
Results
Basic patient characteristics
Among the 79 patients included in the study, 42 (53.2%) were men and 37 (46.8%) were women. The median age of patients at the time of colectomy was 66.1 ± 13.3 years. Table 1 represents the clinical and pathological characteristics at the time of colectomy.
Conventional adenocarcinoma (74.7%) was the predominant histological type of resected tumors followed by mucinous-type adenocarcinoma (22.8%) and signet ring cell adenocarcinoma (2.5%). Grades 1 and 2 tumors (79.7%) predominated over grades 3 and 4 (20.3%) and median tumor size was 4.78 cm ± 1.6 (2 - 8.5) (table 1).
Most patients had stage II-A CC with a frequency of 34.2%. The distribution of patients by stage is shown in table 1.
Most patients presented an absence of lymphatic invasion (82.3%), vascular invasion (88.6%) and perineural invasion (89.9%). In addition, the majority (84.8%) presented a conservation of mismatch repair (MMR) protein expression (table 1).
Table 1: Baseline characteristics of all patients included in the study and comparison between RCC and LCC. Values are presented as mean ± standard deviation (range) or effective (%). The values in bold are those considered significant for a p value less than 0.05.
| Characteristics | Total (N= 79) | RCC (N= 42) | LCC (N= 37) | p-value |
|---|---|---|---|---|
| Age of patients at time of colectomy (years) | 66.1 ± 13.3 (32 - 92) | 67.7 ± 13.2 (40 - 89) | 64.3 ± 13.2 (32 - 92) | 0.26 |
| Sex, Male, N (%) | 42 (53.2%) | 18 (42.9%) | 24 (64.9%) | 0.05 |
| BMI (Kg/m2) | 25.6 ± 4.6 (16.9 – 41.4) | 24.6 ± 4.3 (18.38 - 33.77) | 26.8 ± 4.7 (16.9 - 41.4) | 0.04 |
| Histological type, N (%) Conventional adenocarcinoma Mucinous adenocarcinoma Signet ring cell adenocarcinoma |
59 (74.7%) 18 (22.8%) 2 (2.5%) |
26 (61.9%) 15 (37.5%) 1 (2.4%) |
33 (89.2%) 3 (8.1%) 1 (2.7%) |
0.01 |
| Location, N (%) Cecum Ascending colon Hepatic flexure Transverse colon Splenic flexure Descending colon Sigmoid colon |
16 (20%) 12 (15%) 6 (8%) 8 (10%) |
1 (1%) 3 (4%) 33 (42%) |
||
| Tumor grade, N (%) Grade 1-2 Grade 3-4 |
63 (79.7%) 16 (20.3%) |
29 (69%) 13 (31%) |
34 (92%) 3 (8%) |
0.012 |
| Tumor size (cm) | 4.78 ± 1.6 (2 - 8.5) | 5.1 ± 1.7 (2 - 8.5) | 4.4 ± 1.3 (2.5 - 8) | 0.05 |
| T stage, N (%) T1 T2 T3 T4a T4b |
5 (6.3%) 14 (17.7%) 34 (43.0%) 24 (30.4%) 2 (2.5%) |
0 (0%) 5 (11.9%) 22 (52.4%) 14 (33.3%) 1 (2.4%) |
5 (13.5%) 9 (24.3%) 12 (32.4%) 10 (27.0%) 1 (2.7%) |
0.05 |
| N stage, N (%) N0 N1a N1b N1c N2a N2b |
56 (70.9%) 4 (5.1%) 7 (8.9%) 1 (1.3%) 9 (11.4%) 2 (2.5%) |
26 (61.9%) 2 (4.8%) 4 (9.5%) 0 (0%) 8 (19.0%) 2 (4.8%) |
30 (81.1%) 2 (5.4%) 3 (8.1%) 1 (2.7%) 1 (2.7%) 0 (0%) |
0.13 |
AJCC stage, N (%) Stade I Stade II-A Stade II-B Stade II-C Stade III-A Stade III-B Stade III-C |
15 (19.0%) 27 (34.2%) 13 (16.5%) 1 (1.3%) 2 (2.5%) 13 (16.5%) 8 (10.1%) |
2 (4.8%) 16 (38.0%) 7 (16.7%) 1 (2.4%) 1 (2.4%) 9 (21.4%) 6 (14.3%) |
13 (35.1%) 11 (29.7%) 6 (16.2%) 0 (0%) 1 (2.7%) 4 (10.8%) 2 (5.4%) |
0.03 |
| Lymph node metastases | 1.01 ± 2.05 (0 - 10) | 1.6 ± 2.5 (0 - 10) | 0.4 ± 0.9 (0 - 4) | 0.006 |
| Lymphatic invasion, N (%) | 14 (17.7%) | 10 (23.8%) | 4 (10.8%) | 0.13 |
| Vascular invasion, N (%) | 9 (11.4%) | 5 (11.9%) | 4 (10.8%) | 0.88 |
| Perineural invasion, N (%) | 8 (10.1%) | 4 (9.5%) | 4 (10.8%) | 0.85 |
| Expression of MMR proteins, N (%) Conservation Loss |
67 (84.8%) 12 (15.2%) |
32 (76.2%) 10 (23.8%) |
35 (94.6%) 2 (5.4%) |
0.023 |
| Adjuvant CT, N (%) | 34 (43.0%) | 25 (59.5%) | 16 (43.2%) | 0.15 |
| Recurrence, N (%) | 14 (17.7%) | 8 (19.0%) | 6 (16.2%) | 0.74 |
| Location of distant recurrence, N (%) Hepatic Pulmonary Peritoneal carcinomatosis |
5 (62.5%) 1 (12.5%) 2 (25%) |
3 (60.0%) 1 (20.0%) 1 (20.0%) |
2 (66.7%) 0 (0%) 1 (33.3%) |
0.7 |
| Survival, N (%) Death, N (%) Survival time (months) |
70 (88.6%) 9 (11.4%) 64.8 ± 14.2 (4 - 84) |
35 (83.3%) 7 (16.7%) 60.7 ± 16.1 (4 – 84) |
35 (94.6%) 2 (5.4%) 69.4 ± 10.1 (45 – 83.8) |
0.12 0.01 |
BMI: Body Mass Index; CT: Chemotherapy; LCC: Left-Sided Colon Cancer; MMR: Mismatch Repair; RCC: Right-Sided Colon Cancer.
14 patients (17.7%) presented a recurrence with a predominant hepatic location (62.5%). The 5-year OS of the patients included in our study was 88.6% with a median survival time of 64.8 months. The percentage of deaths was 11.4% (Table 1).
Impact of cancer stage on survival after colectomy
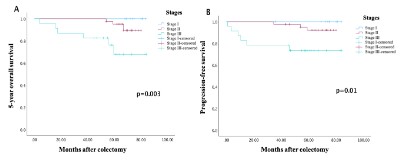
The OS rate at 5 years was 100% for stage I tumors, 92.7% for stage II and 73.9% for stage III, with a significant difference (p = 0.02). The median 5-year survival was 76.7 months for stage I tumors, 66.9 months for stage II and 53.0 months for stage III. The PFS was 74.6 months for stage I, 64.6 months for stage II and 48.2 months for stage III. Patients with stage I CC had higher 5-year OS (figure 2-A) and PFS (figure 2-B) than patients with stage II and stage III, with a significant difference (p=0.003 for OS and p=0.01 for PFS).
Impact of cancer sidedness on survival after colectomy
Clinical and pathological characteristics depending on the sidedness of cancer
Among the patients included, 42 (53.2%) had a right-sided colon cancer (RCC) and 37 (46.8%) had a left-sided colon cancer (LCC). The baseline characteristics of patients with RCC and LCC are shown in table 1.
Patients with RCC were older at the time of colectomy than patients with LCC (67.7 years versus 64.3 years, p=0.26) and the majority were females (57.1% versus 35.1%, p=0.05). A lower BMI was noted in patients with RCC compared to those with LCC (24.6 ± 4.3 vs 26.8 ± 4.7, p = 0.04) (Table 1).
The distribution of the different histological types is shown in Table 1. Conventional adenocarcinoma has a higher tendency to occur in the left colon (89.2% vs 61.9%) rather than in the right colon. However, mucinous-type adenocarcinoma has a higher tendency to occur in the right colon (37.5% vs 8.1%) rather than in the left colon. This trend is statistically significant with a χ² test giving a p-value equal to 0.01. The most frequent location was in the cecum (20%) for RCC and in the sigmoid colon (42%) for LCC (Table 1).
Tumors of patients with RCC had larger size (5.1 ± 1.7 (2 – 8.5) vs 4.4 ± 1.3 (2.5 – 8), p=0.05) and more advanced histological grade (31% vs 8% at grades 3 and 4, p=0.012) than tumors of patients with LCC. The tumors of patients with LCC were mostly stage T1 and T2 (37.8% vs 11.9%, p = 0.05) while those of patients with RCC were mostly stage T3 and T4 (88.1% vs 62.1%, p = 0.05). Patients with RCC had more advanced N stage (38.1% vs 18.9% at stages N1 and N2, p=0.13) and more advanced AJCC cancer stage (38.1% vs 18.9% at stage III, p=0.03) than patients with LCC. Regarding lymph node involvement, the number varied between 0 and 10 positive nodes for RCC, and between 0 and 4 positive nodes for LCC (p=0.006). No significant difference was observed regarding lymphatic, vascular or perineural invasion between RCC and LCC. A higher percentage loss of MMR protein expression was observed in patients with RCC (23.8% vs 5.4%, p=0.023) compared to those with LCC (Table 1).
Among the operated patients, 8 patients (19.0%) with RCC and 6 patients (16.2%) with LCC developed a recurrence after colectomy (p=0.74). Regarding the location of metastases, liver metastases (66.7% vs 60.0%, p=0.7) and peritoneal carcinomatosis (33.3% vs 20.0%, p=0.7) were more frequent for LCC, while pulmonary location was more frequent for the RCC (20.0% vs 0%, p=0.7) (Table 1).
Survival analysis of RCC and LCC after colectomy
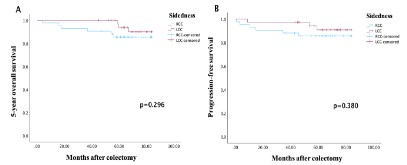
The OS rate at 5 years was 83.3% for RCC and 94.6% for LCC (p=0.1). Patients with LCC showed higher survival time (69.4 ± 10.1 (45 – 83.8) vs 60.7 ± 16.1 (4 – 84), p=0.01) than those with RCC. For PFS, the median duration was 57.7 months for RCC and 66.3 months for LCC. Patients with LCC had a higher 5-year OS (Figure 3A) and PFS (Figure 3B) than patients with RCC (p=0.296 for OS and p=0.380 for PFS).
Impact of cancer grade on survival after colectomy
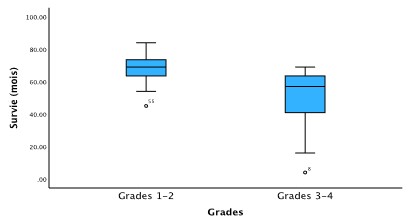
Concerning the distribution by grades and the impact on survival after colectomy, the median 5-year survival was 68.8 months for grades 1 and 2 tumors and 48.7 months for grades 3 and 4 tumors (p<0.001). Figure 4 represents a box plot showing the difference in survival between the 2 groups of grades.
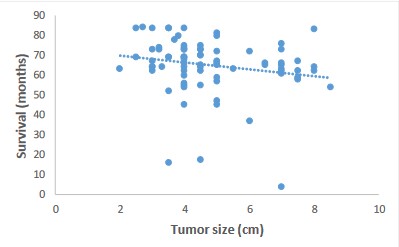
Impact of tumor size on survival after colectomy
Regarding the impact of tumor size at the time of colectomy on survival, we obtained a weak negative correlation with a correlation coefficient equal to -0.2. This result is represented by a scatterplot (Figure 5) showing a non-significant correlation (p=0.09).
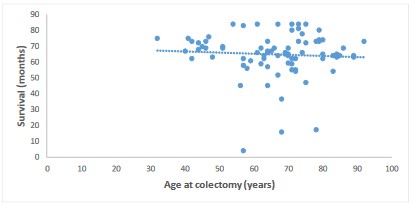
Impact of patient age at colectomy on survival after colectomy
By studying the impact of the patient's age at the time of colectomy on survival, we obtained a moderate negative correlation with a correlation coefficient equal to -0.1. This result is represented by a scatterplot (Figure 6) showing a non-significant correlation (p=0.60).
Impact of different tumor factors on survival after colectomy
The presence of lymphatic, vascular or perineural invasion at the time of colectomy was associated with a lower median survival at 5 years (Table 2). This association was significant for vascular invasion only (p=0.02).
Table 2: Association between survival and various tumor factors. Values are presented as mean ± standard deviation. The values in bold are those considered significant for a p value less than 0.05.
| Variables | Median survival (months) | p-value |
|---|---|---|
| Lymphatic invasion Yes No |
59.8 ± 19.2 65.8 ± 12.9 |
0.58 |
| Vascular invasion Yes No |
59.6 ± 11.4 65.4 ± 14.5 |
0.02 |
| Perineural invasion Yes No |
58.5 ± 9.8 65.5 ± 14.5 |
0.34 |
Impact of taking adjuvant treatment on survival after colectomy
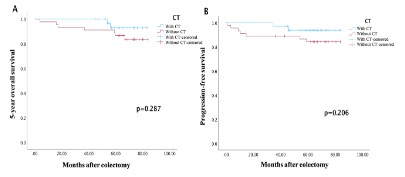
Patients with stage II CC who received adjuvant CT had a higher 5-year OS (Figure 7A) and PFS (Figure 7B) than patients with stage II CC who didn’t receive CT (p=0.287 for OS and p=0.206 for PFS).
Discussion
CC is a deadly disease whose spread has accelerated in recent years. Regarding Lebanon, the country has one of the highest colorectal cancer (CRC) incidence rates in the MENA region. We know little about the epidemiology, pathological features of CC as well as OS and PFS after colectomy in the Middle East and Lebanon. The main objective of our study was to analyze OS at 5 years and PFS after colectomy by studying the impact of several factors.
Our study includes slightly more men than women with a ratio of 1.14. This difference in frequency is already described in Lebanon with a higher prevalence of CC in men.
The majority of CC in our study were in the right colon (53.2%). Indeed, since the 1990s, researchers have begun to observe an increase in the prevalence of RCC compared to LCC in several countries and this can be explained by an improvement in the screening system. However, the low percentage of patients with stage I CC in our study (19.0%) is an indicator that the screening system in Lebanon is not yet fully established, and justifies the need for public health interventions [9].
RCC was more prevalent among women in our study (57.1% vs 35.1%, p=0.005) than LCC. Indeed, women have been shown to have a higher risk of developing RCC than men [10].
Patients with RCC had lower 5-year OS and PFS than those with LCC. But this association between sidedness of tumor and patient survival was not statistically significant (p=0.296 for OS and p=0.380 for PFS). Even though several meta-analyses have demonstrated that RCC has a worse prognosis than LCC, recent studies have shown that there is no significant difference in 5-year OS and time to recurrence between patients with RCC and LCC after curative resection [11,12].
Indeed, a simple comparison of the characteristics of patients with RCC and LCC reveals several significant differences. Patients with RCC in our study had older age at colectomy, lower BMI, more advanced tumor grade, more advanced AJCC stage, higher number of lymph node metastases, higher percentage of lymphatic and vascular invasion and higher loss of MMR protein expression than those with LCC. In fact, patients with RCC have a worse prognosis than those with LCC because they have a worse clinical background. Therefore, to really be able to assess the impact of the sidedness of the tumor on 5-year OS and PFS, it would be necessary to homogenize the history of the two groups.
There was a statistically significant association between the presence of a higher tumor stage at the time of colectomy and a lower 5-year OS (p=0.003) and PFS (p=0.01). We can therefore conclude that tumor stage is a prognostic factor for CC.
Regarding tumor grade, patients with a grade 3 or 4 tumor had lower 5-year OS than those with a grade 1 or 2 tumor with a statistically significant difference (p<0.001). Among our patients, we demonstrated that there was a significant association (p=0.02) between the presence of vascular invasion and the reduction in 5-year OS, which is consistent with the results of several other studies [13]. We can therefore conclude that the tumor grade and the presence of vascular invasion are prognostic factors in CC.
Patients with RCC in our study had a more advanced tumor grade and a higher percentage of vascular invasion than those with LCC, which is consistent with the results of several other studies [11] thus explaining the poorer prognosis of RCC.
We obtained a higher percentage of mucinous-type adenocarcinoma in RCC than LCC. We have not studied the impact of the histological type of cancer on survival, but studies have described a poorer prognosis for mucinous adenocarcinomas [14].
Regarding the size of the tumor, there was a weak correlation between a larger size at the time of colectomy and a lower 5-year OS. But this association was not statistically significant (p=0.09). However, a recent study showed that tumor size was associated with a poor prognosis of CC and was considered a risk factor for recurrence and metastasis [15].
The median age of the patients was 66.1 years. Investigating the impact of patient age at colectomy on survival, we found a moderate correlation between older age and lower 5-year OS, but this association was not statistically significant (p=0.60). This can be explained by the fact that intraoperative complications and postoperative morbidity are higher in elderly patients.
Among the patients included in our study, a total of 34 patients (43.0%) received adjuvant CT. To study the impact of taking adjuvant CT on survival, we focused on patients with stage II CC. Among the 41 patients (51.9%) who had stage II CC, 16 (47.1%) was at high-risk and received adjuvant CT: 10 (62.5%) had stage T4 cancer, 5 (31.3%) had lymphatic or vascular invasion, and 1 (6.3%) had an occlusion. We found that adjuvant CT improves the prognosis of patients with stage II CC, result that is supported by previous studies [16]. However, detailed date on the type of CT have not been taken into consideration in our study.
Loss of expression of MMR proteins was more frequent in RCC (23.8% vs 5.4%, p=0.023) than in LCC, which is consistent with previous studies [11].
Indeed, we did not analyze the impact of the loss of expression of MMR proteins on survival, but a recent study showed that adjuvant CT was a poor prognostic factor for stage II RCC with loss of MMR protein expression and therefore patients should know the MMR protein expression status before receiving adjuvant CT [11].
A significant difference (p=0.04) in BMI between patients with RCC and LCC was observed. Indeed, it has already been shown that patients with RCC have a lower BMI than those with LCC, probably because most patients are women and have an advanced age [11]. In our study, we did not analyze the association between BMI and survival, but studies have shown that a high BMI is a good prognostic factor for CC [11] probably because patients who have a low BMI have very little visceral fat to cover the tumor which can cause it to spread rapidly.
Conclusion
CC is a major public health problem in Lebanon and the incidence is likely to increase over the next few decades. Survival and recurrence after CC resection remain important problems, and early detection is very important. Our study is the first evaluating survival and prognostic factors for resectable CC in the region.
Although there was no significant association between cancer sidedness and survival, patients with RCC have worse prognostic factors. We found that tumor stage, tumor grade as well as vascular invasion at the time of colectomy are prognostic factors that affect survival after colectomy in the Lebanese population. However, further studies would be interesting to carry out on larger samples.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3): 209–49.
- Chatila R, Mansour J, Mugharbil A, Nsouli G, O’Son L, Sayad E, et al. Epidemiology and Survival of Colorectal Cancer in Lebanon: A Sub-National Retrospective Analysis. Cancer Control J Moffitt Cancer Cent. 2021; 28: 10732748211041220.
- H R, Da R. Surgical treatment of colon cancer. Surg Oncol Clin N Am [Internet]. 2006 Jan [cited 2021 Oct 6]; 15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/16389153/
- Benson AB, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2017; 15(3): 370–98.
- Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008; 51(5): 503–7.
- A D, Am B, O B, G R, S M, C L, et al. Net survival in recurrencefree colon cancer patients. Cancer Epidemiol [Internet]. 2019 Aug [cited 2021 Oct 7]; 61. Available from: https://pubmed.ncbi. nlm.nih.gov/31212224/
- S M, Am B, C L, C H, V D, J F. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg [Internet]. 2006 [cited 2021 Oct 7]; 93(9). Available from: https://pubmed.ncbi.nlm.nih.gov/16804870/
- Khachfe HH, Salhab HA, Fares MY, Khachfe HM. Probing the Colorectal Cancer Incidence in Lebanon: an 11-Year Epidemiological Study. J Gastrointest Cancer. 2020; 51(3): 805–12.
- Moussallem M, Jreij M, Yeretzian JS, Asmar MK, Bou-Orm IR. Colorectal cancer screening knowledge and uptake in lebanon: a national survey. Rev Epidemiol Sante Publique. 2022; 70(2): 67–73.
- Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol WJG. 2015; 21(17): 5167–75.
- Yang CY, Yen MH, Kiu KT, Chen YT, Chang TC. Outcomes of rightsided and left-sided colon cancer after curative resection. Sci Rep [Internet]. 2022 [cited 2022 Aug 6]; 12. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9256690/
- Ishizuka M, Shimizu T, Shibuya N, Takagi K, Hachiya H, Nishi Y, et al. Impact of Primary Tumor Location on Survival After Curative Resection in Patients with Colon Cancer: A Meta-Analysis of Propensity Score-Matching Studies. The Oncologist. 2021; 26(3): 196–207.
- Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012; 118(3): 628–38.
- Thota R, Fang X, Subbiah S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. J Gastrointest Oncol. 2014; 5(1): 18–24.
- A B, A DJ, A EM, Smk T, M R. Prognostic Factors of Recurrence and Survival in Operated Patients with Colorectal Cancer. Middle East J Dig Dis [Internet]. 2022 Jan [cited 2023 Jan 18]; 14(1). Available from: https://pubmed.ncbi.nlm.nih.gov/36619730/
- Liu LL, Xiang ZL. Adjuvant chemotherapy improves survival in high-risk stage II colon cancer: a retrospective cohort study. Ther Adv Gastroenterol. 2022; 15: 17562848221137758.