Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 3
Study of insulin-like growth factors and their association with clinic pathological features and survival in colon cancer
Carolina Estevam Martinez¹; Omar Feres²; Joaquim José Ribeiro Rocha²; Jairo Pinheiro da Silva²; Janaina Regina Léllis²; Paulo Cezar Novais4; Victor Cunha Tirapelli²; Maria de Fátima GS Tazima²; Daniela Pretti da Cunha Tirapelli²; Mucio Luiz de Assis Cirino²; Luis Fernando Tirapelli²; Mariângela Orttoboni Brunaldi³; Fernanda Maris Peria¹*
1Division of Clinical Oncology, University Hospital, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, Brazil.
2Division of Surgery and Anatomy, University Hospital, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, Brazil.
3Division of Pathology, University Hospital, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, Brazil.
4Postgraduate Program in Structural and Functional Interations in Rehabilitation, University of Marília, Brazil.
*Corresponding Author : Fernanda Maris Peria
Faculdade de Medicina de Ribeirao Preto-USP Avenida
Bandeirantes, 3900 14049-900 Ribeirao Preto-SP Brazil.
Tel: 55-16-3602-1000;
Email: lab.biomol.cirurgia@fmrp.usp.br
Received : Jun 05, 2023
Accepted : Jun 21, 2023
Published : Jun 28, 2023
Archived : www.jjgastro.com
Copyright : © Maris Peria F (2023).
Abstract
The present study aimed to elucidate the clinicopathological relationship of insulin-like growth factor signaling pathway in patients with advanced colon adenocarcinoma. The relationship of protein expression in this pathway is controversial when associated with the disease prognosis, including its relationship with type II diabetes mellitus. In this study, we examined the expression of IGF 1, IGFBP3 and IRS1 proteins in three groups related to Type II Diabetes Mellitus, and their possible roles in disease prognosis. The clinicopathological characteristics of 91 patients with resected advanced colon adenocarcinoma were retrospectively reviewed, and the expression of IGF1, IGFBP3 and IRS1 were analyzed by immunohistochemistry. IGF1, IGFBP3 and IRS1 expression were observed in 91 patients, respectively. Patients with IGF1 expression have been shown to be associated with poor progression-free survival (p<0.01) and the absence of IGFBP3 expression was associated with shorter overall patient survival (p<0.01). However, in the present study the association of colon adenocarcinoma with IRS1 expression and its relationship with type 2 diabetes mellitus did not present statistically significant. IGF1 signaling may be associated with tumor aggressiveness, and IGFBP3 may show antiproliferative effects on advanced colon adenocarcinoma. Both high IGF1 expression and low IGFBP3 expression represent useful prognostic markers for patients with resected advanced colon adenocarcinoma.
Keywords: Colon cancer; IGF 1; IGFBP 3; IRS 1; Biomarkers.
Citation: Martinez CE, Feres O, Ribeiro Rocha JJ, da Silva JP, Maris Peria F, et al. Study of insulin-like growth factors and their association with clinic pathological features and survival in colon cancer. J Gastroenterol Res Pract. 2023; 3(4): 1145.
Introduction
Cancer is a major challenge among global public health problems both in developed and developing countries and also is a major cause of death. According to the World Health Organization (WHO), the global incidence for 2032 will be 22 million new cases, with the global calculation corrected for sub-registration of 640,000 new cases. Among malignant tumors, Colorectal Cancer (CRC) is ranked third as the most incident in the world and fourth in mortality, with more than 1.4 million new cases annually, second only to lung cancer (1,8 million new cases) and female breast cancer (1.7 million new cases) [1-5].
Despite some hereditary evidences, most cases of colorectal cancer are sporadic Sterpetti et al. and colon adenocarcinoma accounts for more than 90% of the cases, being globally classified as the most incident and second cause of mortality [2,6]. Colon adenocarcinoma is specified as a human tumor of epithelial origin in which the cells function to secrete substances into ducts or cavity lining, being a multifactorial disease influenced by genetic, environmental and lifestyle factors [2]. Among the known etiological factors are those related to diet and western habits, which are sedentary lifestyle and high consumption of red meat, processed foods associated with low intake of fruits and vegetables, which consequently can lead to obesity and metabolic syndrome, which is related to type 2 Diabetes Mellitus (DM2) [6-8].
Due to the metabolic dysfunctions caused by DM2, and its high incidence, this disease may be associated with several other pathologies, as well as its association with the increase in incidence and mortality in several types of cancer, as demonstrated by breast, endometrial, pancreatic and colorectal cancer [9]. In CRC, besides DM2 being related to its incidence – increasing its risk by up to three times-and mortality, epidemiological studies have demonstrated its relationship with a worse prognosis of the disease [7-13].
The molecular mechanisms related to the emergence or that lead to the worst prognosis of CRC are still unclear, but its relationship with some signaling pathways that are also deregulated in DM2 has already been demonstrated. These pathways are involved with cell growth, survival and proliferation, such as the insulin-like growth factor signaling pathway. This signaling pathway regulates energy metabolism and modulates cell proliferation and differentiation, which when deregulated may increase the risk of carcinogenesis, by decreasing genetic stability and repair of DNA incompatibility or by remodeling cellular metabolism that will promote synthesis of proteins, lipids, and nucleotides, and consequently favor the rapid proliferation of cancer cells, being the main determinant in colorectal cancer pathogenesis [11,14].
Initially, early associations of the insulin-like growth factor pathway with cancer were observed in vitro experiments. Thereafter, several epidemiological studies have shown that higher serum IGF-1 levels and lower IGFBO-3 binding is related to the risk of prostate, pancreatic, colorectal, lung and breast cancer, and may be represented as potential biomarkers for cancer [15-26]. In addition, studies have also shown that deregulation of this pathway is associated not only with the risk but also with development, survival, cell invasion (metastasis) and resistance to chemotherapy in CRC [27-32]. However, other studies do not show direct associations of IGF-1 and IGFBP-3 levels with the incidence of cancer [33-35].
Thus, the objective of this study was to analyze the relationship of the insulin-like growth factor signaling pathway through the signaling of the following antibodies, IGF-1, IGFBP-3 and IRS-1, with clinicopathological outcomes and cancer survival rates in advanced colon cancer patients related to type 2 diabetes mellitus, and to analyze these proteins as possible biomarkers for CRC.
Materials and methods
Patients
It is an analytical, cross-sectional study. The sample of the study is of convenience, including ninety patients, raised by CID 10- C18 with colon and rectal adenocarcinoma that resected the tumor in the year 2008 to 2015, and are enrolled in the Proctology Tumor Bank at the HCFMRP-USP. Clinical and sociodemographic information was retrospectively collected through electronic medical record review of all patients participating in the study.
To select the ninety one patients, the following inclusion criteria were applied: Patients enrolled and attended by the Single Health System (SUS); Both sexes; Age higher than 18 years; Histological diagnosis of adenocarcinoma located in the colon; Advanced clinical- surgical stage [stage TNM II (high risk) III and IV]; Oncological treatment according to the current scientific guidelines; Follow- up duration at the HCFMRP-USP Clinical Oncology Outpatient Clinic of at least twenty-four months; They have neoplastic tissues preserved in paraffin; They have stored blood samples; They signed the informed consent form for use of tissue and blood in research. After this sealing the following exclusion criteria were applied: Neoplasia located in the rectum; Diagnosis of other malignancies prevented, except non-melanoma skin tumors; Tumor of the colon and rectum synchronous to the diagnosis; Neoadjuvant treatment; Lynch syndrome; Confirmed diagnosis of type I diabetes mellitus; Patients with malnutrition; Factors that make unfeasible the use of blocks containing the paraffin-shaped tumor tissue.
The patients were divided into three groups: Twenty-three patients with DM2, fifty-two pre-diabetic patients and sixteen non-diabetic patients. Patients`characteristics are show in Table 1. The median age of patients was sixty- one years and six months (range 28-88 years). The majority of patients were female (54,5%), pre-diabetic (57,1%), descending colon and sigmoid location (53,3%), Stage TNM II (high risk) and III classifications (53,9%), positive angiolymphatic invasion (53,9%) and negative for perineural invasion (63,7%), degree of differentiation well and moderately differentiated (87,9%), pathological classification p T3 (69,2%) and p N1 (45,1%), being the majority of patients with metastasis (60,4%). Most patients did not have disease progression and/or recurrence (50,6%) and did not evolve to death (64,8%).
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki (1975). This study was approved by the Ethics and Research Committee of HCFMRP- USP.
Tissue microarrays techniques
The hematoxylin and eosin-stained slides were reviewed by an experienced pathologist in order to delineate the most significant area of each tumor to be used for Tissue Microarrays (TMA). The preparation of the TMA was performed in the laboratory of Oncopatology in the Department of Pathology and Legal Medicine of the Medical School of Ribeirao Preto, Sao Paulo. From each of the ninety blocks of pre-marked donor paraffin, three 2 mm diameter cylinders (one representing the margin and two representing the tumor) were removed and sorted into a paraffin block recipient using the Tissue Microarray Builder Kit) (Histopathology Ltd., Hungary). From each TMA block histological sections of 4 μm thickness were made in a conventional rotary microtome (Leica RM2125RTS, Germany).
Immunohistochemical techniques
One cut was used for each TMA block. All tissue samples were processed according to laboratory routine and with pre-established protocols. For this technique the reactions were performed using the Abcam® ab80436 Expose Mouse and Rabbit Specify HRP / DAB Kit. The sections were dewaxed in xylol and rehydrated in a series of alcohols (95%, 80% and 70%), running water and distilled water. Antigenic recovery was performed using a steam pan with citrate buffer at 10mM for 40 minutes and were cooled for 40 minutes.
Endogenous peroxidase blockade was achieved by solutions from the Abcam® Kit. After this procedure, the primary antibody was incubated for 1 hour at room temperature. The primary antibodies used in this study were IGF1 at 1:50 dilution, anti-human mouse polyclonal antibody (Abcam®, Cambridge, MA, USA, anti-IGF1 antibody 7973), IGFBP3 at 1:10 dilution, anti-human rabbit monoclonal antibody (Abcam®, Cambridge, MA, USA, anti-IRS1 antibody EP263Y ab40777) and IRS1 at 1:50 dilution, anti-human rabbit polyclonal antibody (Abcam®, Cambridge, MA, USA, anti-IFBP3 antibody ab 7001ul400).
After incubations with the primary antibodies, the cuts were incubated with complement for 10 minutes and then incubated with Goat anti-rabbit HPR conjugate, 15-minute amplification system, both from the Abcam® Kit.
The reaction was stained with Diaminobenzidine (DAB) and stained with hematoxylin. The cuts were again hydrated in distilled water and dehydrated in a series of alcohols and xylol. The slides were then covered by cover slip with the aid of Entellan (Merck, Germany).
Immunohistochemical determination
All slides were examined by one pathologist who was blinded to clinical data. To determine the immunoreactivity of IGF1 and IGFBP3 the intensity of the cytoplasm staining pattern was evaluated. The analysis was semi- quantitative through score, scores from 0-3 were given for the staining intensity and the percentage of positive cells as follows: score of 0, no staining is observed, or is observed in less than 10% of the tumor cells; score of 1+, weak staining is detected in 10% or more of the tumor cells; score of 2+, moderate staining is observed in 10% or more of the tumor cells; and score of 3+, strong staining is observed in 10% or more of the tumor cells. Scores of 0 and 1+ were negative for IGF1 and IGFBP3 overexpression, while scores of 2+ and 3+ were positive for IGF1 and IGFBP3 overexpression.
To determine IRS1 immunoreactivity, a specific method described in the literature was used, where the percentage of positive cells was divided into five percentage scores: ≤10% (0), 11-25% (1), 26-50% (2), 51-75% (3) and >75% (4). The intensity of the staining was divided into four intensity scores: no staining (0), light brown (1), brown (2) and dark brown (3). The staining of the positivity was determined by the formula: general scores=percentage score x intensity score. The total score ranged from 0 to 12, with negative expression (0-1) and positive expression (2-12).
Statistical analysis
All associations involving two qualitative variables were performed using the chi-square test. In order to correlate the survival time (or progression) with qualitative variables, Kaplan-Meier curves were constructed for the variables under study and to verify if there was any evidence of differences between the curves, the Log Rank test was used. To relate the survival time with variables of interest, the Cox proportional hazards model was proposed. This model calculates the Hazard Ratio (HR) that provides how much a category has a risk of death (or progression) in relation to the other. To verify if the variables of interest were predictive of metastasis, Odds Ratio (OR) was calculated through gross and adjusted logistic regression. All graphs presented were made with software R, version 3.4.1, and analyzes using SAS 9.2. A P-value <0.05 was considered to represent statistical significance.
Results
Clinicopathological association of IGF1, IGFBP3 and IRS1 expression
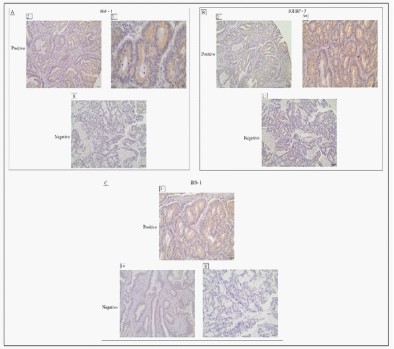
Typical images of positive and negative immunostaining for IGF1 (Figure 1A), IGFBP3 (Figure 1B) and IRS1 (Figure 1C) in colorectal cancer cells. Where 63 cases (69,23%) were positive for IGF1 overexpression, 83 cases (91,21%) were positive for IGFBP3 overexpression and 85 cases (93,4) were positive for IRS1 overexpression.
Table 1 shows the association of clinicopathological characteristics and IGF1, IGFBP3 and IRS1 expression. IGFBP-3 expression had a significant degree of TNM malignant tumors (Fisher´s exact test, p=0.01), presence of metastasis (Fisher´s exact test, p=0.02) and death (Fisher´s exact test, p=0.01). IGF-1 and IRS-1 expression has not yet been found with any variable.
Table 1: Association between IGF1, IGFBP3 and IRS1 expression and clinicopathological factors in resectable colorectal cancer. Type 2 Diabetes Mellitus (DM2); No Diabetes Mellitus (NDM); type 2 Pre-Diabetes Mellitus (PDM).
| Characteristics | IGF1 expression | IGFBP3 expression | IRS expression | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | ||||||||||
| n | % | n | % | p-value | n | % | n | % | p-value | n | % | n | % | p-value | |
| Gender | 0,46 | 0,30 | 0,15 | ||||||||||||
| Female | 17 | 60,7 | 32 | 52,4 | 3 | 37,5 | 47 | 56,6 | 5 | 83,3 | 45,0 | 52,9 | |||
| Male | 11 | 39,3 | 30 | 48,4 | 5 | 62,5 | 36 | 43,4 | 1 | 16,7 | 40,0 | 47,1 | |||
| DM | 0,81 | 0,15 | 0,27 | ||||||||||||
| DM2 | 7 | 25,0 | 16 | 25,8 | - | - | 23 | 27,7 | - | - | 23 | 27,1 | |||
| NDM | 6 | 21,4 | 10 | 15,9 | 1 | 12,5 | 15 | 18,1 | 2 | 33,3 | 14 | 16,5 | |||
| PDM | 15 | 53,6 | 36 | 58,7 | 7 | 87,5 | 45 | 54,2 | 4 | 66,7 | 48 | 56,5 | |||
| Localization | 0,52 | 0,57 | 0,22 | ||||||||||||
| Ascending and transverse (1) | 10 | 35,7 | 27 | 42,9 | 4 | 50,0 | 33 | 39,8 | 1 | 16,7 | 36 | 42,4 | |||
| Descendant and sigmoid (2) | 18 | 64,3 | 36 | 57,1 | 4 | 50,0 | 50 | 60,2 | 5 | 83,3 | 49 | 57,7 | |||
| TNM | 0,62 | 0,01* | 0,30 | ||||||||||||
| II and III | 14 | 50,0 | 35 | 55,6 | 1 | 12,5 | 48 | 57,8 | 2 | 33,3 | 47 | 55,3 | |||
| IV | 14 | 50,0 | 28 | 44,4 | 7 | 87,5 | 35 | 42,2 | 4 | 66,7 | 38 | 44,7 | |||
| Angiolymphatic invasion | 0,07 | 0,21 | 0,85 | ||||||||||||
| No | 9 | 32,1 | 33 | 52,4 | 2 | 25,0 | 40 | 48,2 | 3 | 50,0 | 39 | 45,9 | |||
| Yes | 19 | 67,9 | 30 | 47,6 | 6 | 75,0 | 43 | 51,8 | 3 | 50,0 | 46 | 54,1 | |||
| Perineural invasion | 0,18 | 0,40 | 0,47 | ||||||||||||
| No | 15 | 53,6 | 43 | 68,3 | 4 | 50,0 | 54 | 65,1 | 3 | 50,0 | 55 | 64,7 | |||
| Yes | 13 | 46,4 | 20 | 31,8 | 4 | 50,0 | 29 | 34,9 | 3 | 50,0 | 30 | 35,3 | |||
| Histology | 0,79 | 0,97 | 0,35 | ||||||||||||
| Well and moderately differs (1) | 25 | 89,3 | 55 | 87,3 | 7 | 87,5 | 73 | 88,0 | 6 | 100,0 | 74 | 87,1 | |||
| Little differentiated and undifferentiated (2) | 3 | 10,7 | 8 | 12,7 | 1 | 12,5 | 10 | 12,1 | - | 0,0 | 11 | 12,9 | |||
| T category | 0,29 | 0,11 | 0,62 | ||||||||||||
| 2 | 2 | 7,1 | 6 | 9,5 | - | - | 8 | 9,6 | - | - | 8 | 9,4 | |||
| 3 | 17 | 60,7 | 46 | 73,0 | 4 | 50,0 | 59 | 71,1 | 4 | 66,7 | 59 | 69,4 | |||
| 4 | 9 | 32,1 | 11 | 17,5 | 4 | 50,0 | 16 | 19,3 | 2 | 33,3 | 18 | 21,2 | |||
| N category | 0,88 | 0,62 | 0,96 | ||||||||||||
| 0 | 4 | 14,3 | 7 | 11,1 | - | - | 11 | 13,6 | 1 | 16,7 | 10 | 11,8 | |||
| 1 | 13 | 46,4 | 28 | 44,4 | 5 | 62,5 | 36 | 43,3 | 3 | 50,0 | 38 | 44,7 | |||
| 2 or 3 | 11 | 39,3 | 27 | 42,9 | 3 | 37,5 | 35 | 42,2 | 2 | 33,3 | 36 | 42,4 | |||
| X | - | - | 1 | - | - | 1 | 1,2 | - | - | 1 | 1,2 | ||||
| Metastasis | 0,97 | 0,02 | 0,24 | ||||||||||||
| No | 11 | 39,3 | 25 | 39,7 | - | - | 36 | 43,4 | 1 | 16,7 | 35 | 41,2 | |||
| Yes | 17 | 60,7 | 38 | 60,3 | 8 | 100,0 | 47 | 56,6 | 5 | 83,3 | 50 | 58,8 | |||
| Progression and/or recurrence | 0,20 | 0,97 | 0,38 | ||||||||||||
| No | 17 | 60,7 | 29 | 46,0 | 4 | 50,0 | 42 | 50,6 | 2 | 33,3 | 44 | 51,8 | |||
| Yes | 11 | 39,3 | 34 | 54,0 | 4 | 54,0 | 41 | 49,4 | 4 | 66,7 | 42 | 48,2 | |||
| Death | 0,31 | 0,01* | 0,09 | ||||||||||||
| No | 16 | 57,1 | 43 | 68,3 | 2 | 25,0 | 57 | 68,7 | 2 | 33,3 | 57 | 67,1 | |||
| Yes | 12 | 42,9 | 20 | 31,8 | 6 | 75,0 | 6 | 31,3 | 4 | 66,7 | 28 | 32,9 | |||
In the three groups studied, it is possible to verify that patients with DM2 (25.8%; 27.7%; 27.1%) and pre-diabetic patients (58.7%; 54.2%; 56.5%) had more patients with overexpression of IGF1, IGFBP3 and IRS1 proteins, respectively, compared to non-diabetics (15.9%; 18.1%; 16.5%).
Association of protein expression with prognosis of the disease under study
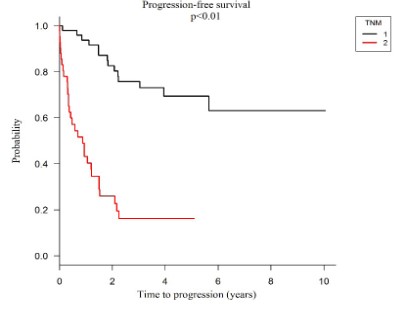
Progression-free survival: Kaplan-Meier survival analyzes, and the COX regression model showed significantly worse progression-free survival in patients classified as TNM IV compared to TNM II high risk and TNM III. Patients with TNM IV classification had 43%, 26% and 16% of progression-free survival at 1, 2 and 5 years respectively, while those with the TNM II high risk and TNM III classification presented 94%, 83%, 69% progression-free survival at 1, 2 and 5 years respectively, as seen in graph 1 (HR=6.24; p<0.01).
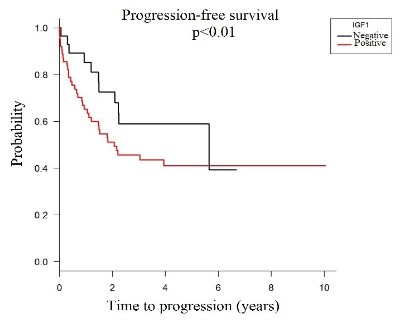
In addition, patients who showed IGF-1 protein overexpression showed lower progression-free survival when compared to patients who did not show IGF-1 protein expression (HR=1.58; p <0.01), graph 2.
Overall survival
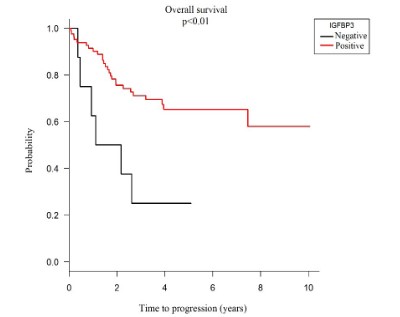
Through Kaplan - Meier survival analysis and COX regression model, overall survival showed a statistically significant relationship with IGFBP-3 protein overexpression with a life expectancy of 65.0% at 5 years. And when this protein is negatively expressed, the 5-year life probability decreases to 25.0% (HR = 3.49; p <0.01), as shown in graph 3.
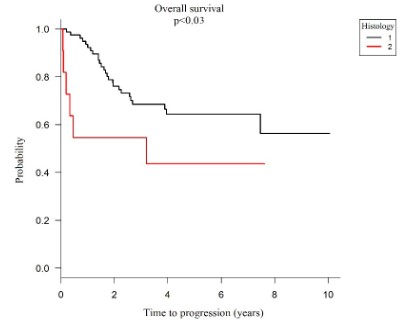
When analyzing the histological type of the tumor, it was found that when the tumor histology classification is well and moderately differentiated (1) there is 94% overall survival in 1 year (p=0.03), when it is classified as a poor tumor. Differentiated and undifferentiated (2) the probability of 1-year overall survival decreases to 55%, showing that patients with poorly differentiated and undifferentiated histological adenocarcinomas have significantly worse overall survival than those with well and moderately differentiated adenocarcinomas (HR=2.61; p<0.01) as shown in graph 4.
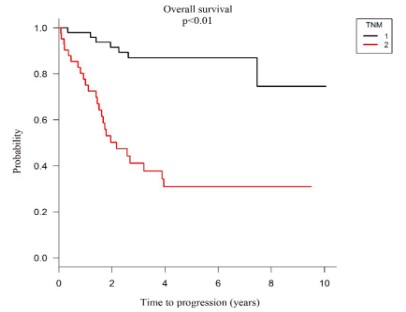
Similar to progression-free survival, when analyzing the classification of malignant tumors in overall survival, it was possible to observe that patients with metastasis, those with TNM IV classification, have significantly lower overall survival, 31.0% in 5 years than those without the disease spread to other organs, 87.0% in 5 years (p <0.01), as seen in graph 5.
In the analysis of variables related to the presence of metastasis, there was an association with IGFBP3 protein (p=0.02), presence of perineural invasion (HR=2.80; p=0.02) and pathological classification pT (p<0 .01).
Discussion
The present study analyzed the immunohistochemical expression of IGF1, IGFBP3 and IRS1 with clinicopathological variables and the correlation with progression-free survival and overall survival in 91 patients with advanced colon adenocarcinoma. IGF1 expression had a significant association with progression - free survival, in which patients who had positive IGF1 had significantly poorer survival compared to the IGF1 - negative group. This finding suggests that IGF1 and its signaling system are related to worse prognosis in advanced colon adenocarcinoma, including that its higher bioavailability may be related to tumor aggressiveness, as reported by other studies [7,11,38-42].
IGF1 bioavailability is regulated by a family of six IGF-binding proteins (IGFBP), of which IGFBP3 is the major IGF carrier protein. It has been shown in previous studies that the presence of IGFBP3 makes IGF1 less bioavailable because it binds to it and blocks its ability to bind to its receptors, reducing its activation of downstream reactions in the signaling pathway. Its function is controversial. In this study, a favorable survival was observed for the positive IGFBP3 group, with statistical significance. These findings suggest that IGFBP3 may have antiproliferative effects related to advanced colon adenocarcinoma, which has already been seen in other studies where patients showing IGF1 positive and IGFBP3 negative expression have a worse prognosis / disease progression [40-51].
The IRS1 protein did not demonstrate statistical significant data in the studied group. IRS proteins are from a family of cytoplasmic adapter proteins that transmit signals from insulin and IGF1 receptors to induce a cellular response, but it is noteworthy that no studies to date have been performed with advanced colon adenocarcinoma and that related studies are inconclusive. When analyzing the expression of IRS1 in the total of patients, 85 patients (93,4%) presented positive expression for IRS1 and only 6 (6,6%) negative expression, which possibly shows that its expression may be related to the risk of advanced colon adenocarcinoma, since most patients presented overexpression, as shown in the literature [33,34,52-58].
By analyzing the diabetic, pre-diabetic and non-diabetic patient groups, it was found that patients with type 2 diabetes mellitus and pre-diabetes tend to have a lower progression-free survival compared to the non-diabetic group. In diabetic studies, studies have already confirmed that patients with type 2 diabetes mellitus showed a worse prognosis in relation to cancer, in this study it is presented that this relationship occurs and that it is also related to the insulin-like growth factor signaling pathway, where it is deregulated in the studied pathologies [4,59].
Conclusion
Further studies are needed to elucidate the role and relationship of IRS1 with advanced colon adenocarcinoma and type 2 diabetes mellitus. IGF1 signaling may be associated with tumor aggressiveness, and IGFBP3 may show antiproliferative effects on advanced colon adenocarcinoma. Both high IGF1 expression and low IGFBP3 expression represent useful prognostic markers for patients with resected advanced colon adenocarcinoma.
References
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of Cancer Incidence in the United States: Burdens Upon Aging, Changing Nation. Journal of Clinical Oncology. 2009; 17: 2758-2765.
- Jung HJ, Suh Y. Regulation of IGF -1 signaling by microRNAs. Frontiers in Genetics. Disponível em.
- DENG H, WANG JM, LI M, TANG R, TANG K, et al. Long non-coding RNAs: New biomarkers for prognosis and diagnosis of colon cancer. Tumor Biology. 1-11, 2017 June.
- HASONO K, ENDO H, TAKAHASHI H, SUGIYAMA M, SAKAI E, et al. Metformin Suppresses Colorectal Aberrant Crypt Foci in a Shortterm Clinical Trial. Cancer Prev Rev. 2010; 3.
- WANG P, ZHOU Y, MEI Q, ZHAO J, HUANG L, et al. PPA1 regulates tumor malignant potential and clinical outcomes of colon adenocarcinoma through JNK pathways. Oncotarget. 2017; 8: 58611-58624.
- STERPETTI AV, SAPIENZA P. Adenocarcinoma in the Transposed Colon: High Grade Active Inflammation Versus Low Grade Chronic Inflammation. Eur J Surg Oncol. 2019; 45: 1536-1541.
- SHLOMAI G, NEEL B, LEROITH D, GALLAGHER EJ. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacoterapy. Journal of Clinical Oncology. 2016; 34: 4261-4269.
- JOSHI P, JOSHI RK, KIM WJ, LEE SA. Insulin- like growth factor- 1, IGF- binding protein- 3, C- peptide and colotectal cancer: A case-control study. Journal of Cancer Prevention. 2015; 16: 3735-3740.
- OZOUGWU JC, OBIMBA KC, BELONWU CD, UNAKALAMBA CB. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. Journal of Physiology and Pathophysiology. 2013; 4: 46-57.
- SHARMA A, HANYANN N, KUMAR A, TELI K, RANDHAWA J, et al. Colorectal Cancer: Histopathologic differences in tumor characteristics between patients with and without diabetes. Clinical Colorectal Cancer. 2014; 13: 54-61.
- YANG J, NISHIHARA R, ZHANG X, OGINO S, QIAN ZR. Energy sensing pathways: Bridging type 2 diabetes and colorectal cancer? J. Diabetes Complications. 2017; 31: 1228-1236.
- GIOVANNUCCI E, HARLAN DM, ARCHER MC, BERGENSTAL RM, GAPSTUR SM, et al. Diabetes and cancer: A consensus report. Diabetes care. 2010; 33: 1674-1685.
- DE KORT S, MASCLEE AAM, SANDULEANU S, WEIJENBERG MP, VAN HERK-SUKEL MPP, et al. Higher risk of colorectal cancer in patients with newly diagnosed diabetes mellitus before the age of colorectal cancer screening initiation. Sci Rep. 2017; 7: 46527.
- Jung HJ, Suh Y. Regulation of IGF -1 signaling by microRNAs. Frontiers in Genetics. Disponível em. 2015; 5.
- CHAN JM, STAMPFER MJ, GIOVANNUCCI E, GRANN PH, MA J, et al. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science, 1998; 279: 563–566.
- WOLK A, MANTZOROS CS, ANDERSSON SO, BERGSTRO MR, SIGNORELLO LB, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J. Natl. Cancer Inst. 1998; 90: 911-915.
- HARMAN S, METTER E, BLACKMAN M, LANDIS P, CARTER H. Serum levels of insulin-like growth factor 1 (IGF-I), IGF-II, IGF-binding protein-3, and prostate- specific antigen as predictors of clinical prostate cancer. J. Clin. Endocrinol. Metab. 2000; 85: 4258-4265.
- MA J, POLLAK MN, GIOVANNUCCI E, CHAN JM, TAO T, et al. Prospective study of colorectal cancer risk in men and plasma levels of Insulin-Like Growth Factor (IGF)-1 and IGF-binding protein-3. J. Natl. Cancer Inst. 1999; 91: 620-625.
- GIOVANNUCCI E, POLLAK MN, PLATZ EA, WILLETT WC, STAMPFER MJ, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomark Prev. 2000; 9: 345-349.
- RENEHAN AG, PAINTER JE, O’HALLORAN D, ATKIN WS, POTTEN CS, et al. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000; 85: 3402-3408.
- MANOUSOS O, SOUGLAKOS J, BOSETTI C, TZONOU A, CHATZIDAKIS V, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999; 83: 15-17.
- YU H, SPITZ MR, MISTRY J, GU J, HONG WK, et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: A case-control analysis. J Natl Cancer Inst. 1999; 91: 151-156.
- HANKINSON SE, WILLETT WC, COLDITZ GA, HUNTER DJ, MI-CHAUD DS, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998; 351: 1393-1396.
- BRUNING PF, VAN DOORN J, BONFRER JMG. Insulin-like growth-factor- binding protein 3 is decreased in early-stage operable premenopausal breast cancer. Int J Cancer. 1995; 62: 266-270.
- BAXTER RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000; 278: 967-76.
- HIRAKAWA T, YASHIRO M, MURATA A, HIRATA K, KIMURA K, et al. IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer. BMC Cancer. 2013; 13: 392.
- GIOVANNUCCI, E. Insulin, Insulin-Like growth factors and colon cancer: A review of evidence. The journal of nutrition. 2001; 131: 3109S- 3120S.
- WEBER MM, FOTTNER C, LIU SB, JUNG MC, ENGELHARDT D, et al. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002; 95: 2086-2095.
- SEKHARAM M, ZHAO H, SUN M, FANG Q, ZHANG Q, et al. Insulin-like growth factor 1 receptor enhances invasion and induces resistance to apoptosis of colon cancer cells through the Akt/ Bcl-x (L) pathway. Cancer Res. 2002; 63: 7708-7716.
- DALLAS NA, XIA L, FAN F, GRAY MJ, GAUR P, et al. Chemoresistant Colorectal Cancer Cells, the Cancer Stem Cell Phenotype and Increased Sensitivity to Insulin-like Growth Factor-I Receptor Inhibition. Cancer Research, 2009; 69: 1951-1957.
- WU Y YAKAR S, ZHAO L, HENNIGHAUSEN L, LEROITH D. Circulating Insulin like Growth Factor-I Levels Regulate Colon Cancer Growth and Metastasis. Cancer Research. 2002; 62: 1030-1035.
- VOLKOVA E, ROBINSON BA, WILLIS J, CURRIE MJ, DACHS G. Marginal effects of glucose, insulin and insulin-like growth factor on chemotherapy response in endothelial and colorectal cancer. Oncology letters. 2014; 7: 311-320.
- ESPOSITO DL, ARU F, LATTANZIO R, MORGANO A, ABBONDANZA M, et al. The Insulin Receptor Substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS One. 2012; 7: e36190.
- KORNMANN M, MARUYAMA H, BERGMANN U, TANGVORANUNTAKUL P, BEGER HG, et al. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998; 58: 4250-4254.
- HELLAWELL GO, TURNER GDH, DAVIES DR, POULSOM R, BREWSTER SF, et al. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002; 62: 2942-2950.
- YANCIK, R; RIES, LG. Cancer in the aged: An epidemiologic perspective on treatment issues. Cancer, December 1, supplement. 1991.
- LUO J, WEN Q, LI J, XU L, CHU S, et al. Increased expression of IRS-1 is associated with lymph node metastasis in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2014; 7: 6117-6124.
- GAO Y, KATKI H, GRAUBARD B, POLLAK M, MARTIN M, et al. Serum IGF1, IGF2, and IGFBP 3 and risk of advanced colorectal adenoma. Int J cancer. 2012; 131: E105-E113.
- NETO JDRT, TEIXEITA FR, PRUDENTE ACL, SILVINO CJ, ARCIERE JS, et al. Estudo Demográfico do Câncer de Cólon e Reto no Estado de Sergipe. Rev bras Coloproct .Abril/junho. 2008.
- GIOVANNUCCI E, POLLAK MN, PLATZ EA, WILLETT WC, STAMPFER MJ, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000; 9: 345-349.
- RENEHAN, AG; PAINTER, JE; ATKIN, WS; POTTEN, CS; SHALET, SM; O’DWYER, ST. High-risk colorectal adenomas and serum insulin-like growth factors. Br J Surg. 2001; 88: 107-113.
- TERAMUKAI S, ROHAN T, LEE KY, EGUCHI H, ODA T, et al. Insulin-Like Growth Factor (IGF)-I, IGF-binding protein-3 and colorectal adenomas in Japanese men. Jpn J Cancer Res. 2002; 93: 1187-1194.
- RINALDI S, CLEVELAND R, NORAT T, BIESSY C, ROHRMANN S, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010; 126: 1702-1715.
- MA J, POLLAK MN, GIOVANNUCCI E, CHAN JM, TAO Y, et al. Prospective study of colorectal cancer risk in men and plasma levels of Insulin-Like Growth Factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999; 91: 620-625.
- KAAKS R, TONIOLO P, AKHMEDKHANOV A, LUKANOVA A, BIESSY C, et al. Serum C-peptide, Insulin-Like Growth Factor (IGF)-I, IGFbinding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000; 92: 1592-1600.
- NOMURA AM, STEMMERMANN GN, LEE J, POLLAK MN. Serum insulin-like growth factor I and subsequent risk of colorectal cancer among Japanese- American men. Am J Epidemiol. 2003; 158: 424-431.
- TSILIDIS KK, BRANCATI FL, POLLAK MN, RIFAI N, CLIPP SL, et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2010; 21: 1-10.
- KEKU, TO; LUND, PK; GALANKO, J; SIMMONS, JG; WOOSLEY, JT; SANDLER, RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005; 14: 2076-2081.
- LE MARCHAND L, WANG H, RINALDI S, KAAKS R, VOGT TM, et al. Associations of plasma C-peptide and IGFBP-1 levels with risk of colorectal adenoma in a multiethnic population. Cancer Epidemiol Biomarkers Prev. 2010; 19: 1471-1477.
- RENEHAN AG, PAINTER JE, O’HALLORAN D, ATKIN WS, POTTEN CS, et al. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000; 85: 3402-3408.
- SCHOEN RE, WEISSFELD JL, KULLER LH, THAETE FL, EVANS RW, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005; 129: 464-475.
- SHAW, LM. The Insulin Receptor Substrate (IRS) proteins: At the intersection of metabolismo and cancer. University of Massachusetts Medical School; Worcester, MS, USA. 2011; 1750-1756.
- EWTON DZ, KANSRA S, LIM S, FRIEDMAN E. Insulin-like growth factor-I has a biphasic effect on colon carcinoma cells through transient inactivation of forkhead1, initially mitogenic, then mediating growth arrest and differentiation. Int J Cancer. 2002; 98: 665-673.
- HOWARTH GS. Insulin-like growth factor-I and the gastrointestinal system: therapeutic indications and safety implications. J Nutr. 2003; 133: 2109-2112.
- REINMUTH N, FAN F, LIU W, PARIKH AA, STOELTZING O, et al. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab Invest. 2002; 82: 1377-1389.
- YANG GY, XU KS, PAN ZQ, ZHANG ZY, MI YT, et al. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008; 99: 879-887.
- YOKOYAMA S, TAKIFUJI K, HOTTA T, MATSUDA K, TOMINAGA T, et al. Moderately differentiated colorectal adenocarcinoma as a lymph node metastatic phenotype: Comparison with well differentiated counterparts. BMC Surgery. 2010; 10: 8.
- BOMMER GT, FENG Y, IURA A, GIORDANO TJ, KUICK R, et al. IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype. J Biol Chem. 2010; 285: 1928-1938.
- KIM T, KIM T, CHOI S, KO H, PARK D, et al. Combination of BEZ235 and Metformin has synergistic effect on cell viability in colorectal cancer cells. Dev. Reprod. 2018; 22: 133-142.