Journal of Gastroenterology Research and Practice
Case Report- Open Access, Volume 3
A case of alcoholic liver failure with leukemoid reaction
Xiaohao Wang; Shiying Li; Lu Zhang; Dachuan Cai*
Department of Infectious Diseases, Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Institute for Viral Hepatitis, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China.
*Corresponding Author : Dachuan Cai
Department of Infectious Diseases, Key Laboratory of
Molecular Biology for Infectious Diseases (Ministry
of Education), Institute for Viral Hepatitis, the Second
Affiliated Hospital, Chongqing Medical University,
Chongqing, China.
Email: cqmucdc@cqmu.edu.cn
Received : Mar 27, 2023
Accepted : Apr 25, 2023
Published : May 02, 2023
Archived : www.jjgastro.com
Copyright : © Cai D (2023).
Abstract
Alcoholic liver failure with leukemoid reactions is a relatively rare disease, which is prone to misdiagnosis or missed diagnosis in clinical diagnosis. The diagnosis of the disease mainly relies on histomorphology and immunohistochemistry. Currently, alcohol withdrawal, gluco-corticoid therapy and artificial liver are the main treatment methods, which all require long-term close follow-up.
Citation: Wang X, Li S, Zhang L, Cai D. A case of alcoholic liver failure with leukemoid reaction. J Gastroenterol Res Pract. 2023; 3(3): 1139.
Introduction
A 24-year-old male was admitted due to jaundice, fatigue, decreased appetite, abdominal distension and abdominal pain for 20 days. The patient denied excessive alcohol intake, chronic liver diseases and other medical histories. He took some herbal remedies for several days and visited a local clinic, where laboratory examination revealed the following biochemical results: leukocytosis 24.59 × 109/L, with absolute neutrophil count 20.28 × 109/L, hemoglobin 145 g/L, albumin 27.8 g/dL, AST 100 U/L, ALT 33.7 U/L, alkaline phosphatase 397.8 U/L, γ-glutamyl transferase 1229.1 U/L, total bilirubin 253.8 μmol/L, PTA 47.1%, INR 1.66. A computed tomography (CT) scan revealed an enlarged liver. After treatment with liver-protective drugs combined with antibiotics, there is no improvement in liver function. Then the patient was transferred to our hospital for further evaluation and management.
On admission to our hospital, vital signs were notable for temperature 36.9°C (98.1°F), blood pressure 129/77 mmHg, pulse 109 bpm, and respiratory rate 20/min. Physical examination showed generalized icterus. The liver edge and the spleen edge descended 1.5 cm and 3 cm below the costal margin, respectively, and edema was also noticed in the lower limb.
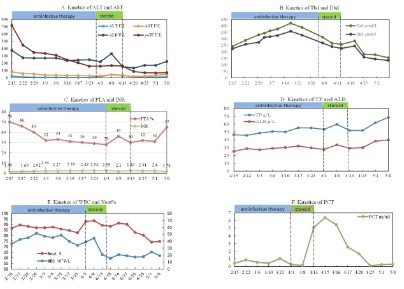
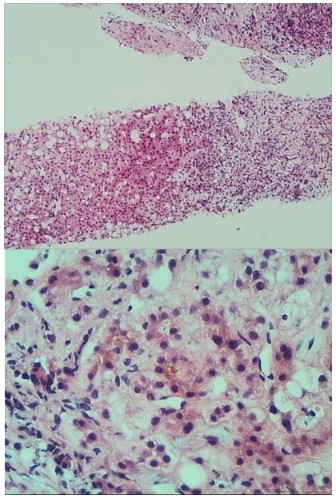
Laboratory tests revealed an ALP level of 376 U/L, an ALT level of 25 U/L, an AST level of 83 U/L, a gamma-glutamyl transferase level of 717 U/L, a total bilirubin level of 243.2 μmol/L, a direct bilirubin level of 224.9 μmol/L, an albumin level of 25.3 g/L and a serum creatinine level of 42.3 mmol/L. Viral serologies (hepatitis A, B, C, D, and E viruses, cytomegalovirus, parvovirus B19, rubella virus and Herpes simplex virus) and autoimmunity markers were all negative. Serum immunoglobulin (Ig) G level, IgA and IgM levels were normal. His serum alpha-1 anti-trypsin and ceruloplasmin levels were normal. Full blood count (FBC) revealed neutrophilia (WBC 36.16 x 109/L, Neut 31.34 x 109/L) with a haemoglobin level of 118 g/L and platelet count (405 x 109/L). Serum level of C-reactive protein was 72.06 mg/L and procalcitonin (PCT) was 0.45 ng/mL. The PTA and INR level were 50% and 1.59, respectively. A computed tomography (CT) scan of the lung shown the sign of pneumonia and hydrothorax. Ultrasonography of the portal vein revealed signs of cavernous changes and splenic vein dilation. A Magnetic Resonance Imaging (MRI) scan of the abdomen revealed an enlarged liver, hepatic parenchymal edema, the edema of gallbladder wall and small amount of ascites. We suspected that the dysfunction of the liver was caused by traditional Chinese medicine which the patient took at the early period of the disease. The patient was treated with hepatoprotective drugs, including acetylcysteine for injection, magnesium isoglycyrrhizinate injection and so on. Because of the high level of neutrophil and the inflammatory lesion in the lung, imipenem-cilastatin was also prescribed to the patient. With these treatments, biochemical parameters such as ALT and AST values improved (Figure 1A). However, the liver function continued to deteriorate, with the increasing level of TBil, DBil (Figure 1B) and INR (Figure 1C). The albumin was still at a low level (Figure 1D). On the other hand, even treatment with imipenem-cilastatin, the leukocytosis, neutrophil count and procalcitonin continued to increase (Figure 1E and Figure 1F). In addition, the patient had no fever or respiratory symptoms. We speculated that the high level of leukocytosis and neutrophil was not totally related to inflammation and this was maybe relevant to the disorder of the hematopoietic system. Then, the bone marrow biopsy, flow cytometry, BCR/ABL gene and Myeloproliferative Neoplasms (MPN) genetic testing were performed. However, these tests shown no obvious abnormalities. The hematopoietic disorder was excluded. The neutrophilia was regarded as a leukemia-like reaction. However, the reason for this phenomenon was still unknown. So, we conduct consultations in conjunction with multiple departments, including radiology department, pathology department, gastroenterology department and rheumatology and immunology department. After the consultation, the sinusoidal obstruction syndrome, Budd-Chiari syndrome and immune system diseases were also excluded. To identify the cause of the disease in this patient, a transjugular liver biopsy was performed. The liver tissue biopsy was sent to Pathology Department of our hospital, the Kingmed Diagnostics, and the Beijing Friendship Hospital. Pathological findings showed characteristic features of alcoholic hepatitis. We comunicat ed with the patient again and again, and finally the patient admitted that he had a history of alcohol intake for about 6 years. Moreover, in the recent two years, the alcohol intake was much higher than before with about 100 g/d. Combined with the patient’s medical history and pathological examination results (Figure 2), and according to the Guidelines for Prevention and Treatment of Alcoholic Liver Disease 2018 Edition [1], the patient was clearly diagnosed with alcoholic hepatitis. After the review of related literature, we found that, it was rare that a patient developed alcoholic liver disease and leukemia-like reactions at the same time [2]. As regard to the alcoholic hepatitis, corticosteroids were applied to the patients in addition to the hepatoprotective drugs such as adenosylmethionine, Magnesium Isoglycyrrhizinate Injection and so on. The patient was first treated with methylprednisolone 40 mg (2 times/d) for 3 days. Then the dose of methylprednisolone was reduced to 40 mg once a day for 1 week. Finally, the corticosteroids were taken orally for a period of time (gradually reducing the dose of corticosteroids). After using corticosteroids for about 1 week, the patient’s liver was significantly smaller than before, the liver edge descended 5 cm below the costal margin. The level of TBil was reduced obviously (Figure 1B). The PTA and albumin value gradually increased after treatment (Figure C, Figure D). It was clear that the treatment was effective, which was consistent with the diagnosis. The patient’s Glasgow Alcoholic Hepatitis Score (GAHS) reached 11 points, which indicated a poor prognosis. After using corticosteroids for about 2 weeks, the patient developed a fever. A chest CT scan revealed scattered patchy ground-glass opacities and consolidations in both lungs. Serological test for respiratory viruses (including influenza, respiratory syncytial virus, adenovirus, and Mycoplasma pneumoniae) were negative. Repeated blood, and ascites samples were culture also negative. Laboratory tests showed a high level of PCT 6.07 ng/ml. Oral fungal smear are positive. We suspected that he had hospital-acquired pneumonia, imipenem-cilastatin, vancomycin, and caspofungin were applied to the patient to treat pneumonia. After treatment for about 3 weeks, the lesions in the Lungs were significantly less than before, and the liver function improved further. Finally, the patient was discharged from the hospital.
In rare cases, alcoholic hepatitis can indeed be combined with leukemia-like reactions [2]. The mechanism is not yet clear. It has been reported that in patients with alcoholic liver disease, long-term drinking can reduce the defensive ability of the intestinal mucosa and increase intestinal toxins, which enters the blood circulation and stimulates monocytes to secrete IL1, IL6, IL8, etc. [3-7]. This is also consistent with this case, the patient’s IL1, IL6, IL8 is elevated. Especially IL8, as a leukocyte chemotactic factor, can cause an increase in white blood cell count [7-8]. Ethanol induces an increase of endotoxin levels, which is transported to the liver and cleared by Kupffer cells. The products produced by Kupffer cells can promote the infiltration of white blood cells into liver tissues [8]. In this case, the neutrophil infiltration in the portal area and the lobules under the pathological section microscope were observed, which meets to this speculation. Although the mechanism of this phenomenon has not yet been determined, we hope that this report can provide some reference for subsequent researchers.
References
- Guidelines of prevention and treatment for alcoholic liver disease: a 2018 update. National Workshop on Fatty Liver and Alcoholic Liver Disease Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. 2018; 34: 939-946.
- Haque AU, Aan NU. Leukemoid reaction. Unusual causes. Int J Pathol. 2010; 8: 39-40.
- Fujimato M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, et al. Plasma endotoxin and serum cvtokine 1evel in patients with alkoholic hepatitis relation to sevetity of liver disturbance. Alcohol Clin Exp Res. 2000; 24: 48-54.
- Sheron N, Bird G, Goka J, Alexander G, Williams R. Elevated plasma interleukin-6 and increased severity and mortality in alcoholic hepatitis. Clin Exp Immunol. 1991; 84: 449-453.
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000; 24: 48S-54S.
- Homann C, Benfield TL, Graudal NA,Garred P. Ncoperin and interleukin-8-prognosis in alcohol-induced cirrboais. Liver. 2000; 20: 442-449.
- Huang YS, Wu JC, Chang FY, Lee SD. Interleukin-8 and alcoholic liver disease. Zhonghua Yi Xue Za Zhi (Taipei). 1999; 62: 395-401.
- Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin ExpRes. 2005; 29: 172S-179S.