Journal of Gastroenterology Research and Practice
Case Report- Open Access, Volume 3
Atezolizumab plus bevacizumab followed by lenvatinib in a case of unresectable hepatocellular carcinoma with Vp4 and child-pugh B
Akina Yoshikawa1; Daisaku Fujimoto1; Ai Miyagi1; Seiko Okushi1,2; Junko Miyagi1; Moriaki Yamanaka3; Toshihito Tanahashi1,2*
1Department of Internal Medicine, Local Incorporated Administrative Agency, Tokushima Prefecture Naruto Hospital, 32 Kurosaki Aza Kotani Muya-cho, Naruto-city, Tokushima 772-8503, Japan.
2Health Management Center, Local Incorporated Administrative Agency, Tokushima Prefecture Naruto Hospital, 32 Kurosaki Aza Kotani Muya-cho, Naruto-city, Tokushima 772-8503, Japan.
3Department of Radiology, Local Incorporated Administrative Agency, Tokushima Prefecture Naruto Hospital, 32 Kurosaki Aza Kotani Muya-cho, Naruto-city, Tokushima 772-8503, Japan.
*Corresponding Author : Toshihito Tanahashi
Department of Internal Medicine, and Health Management
Center, Local Incorporated Administrative Agency,
Tokushima Prefecture Naruto Hospital, 32 Kurosaki Aza
Kotani Muya-cho, Naruto-city, Tokushima 772-8503, Japan.
Tel: +81-88-683-0011; Email: tanahashi@kuh.biglobe.ne.jp
Received : Mar 08, 2023
Accepted : Mar 22, 2023
Published : Mar 31, 2023
Archived : www.jjgastro.com
Copyright : © Tanahashi T (2023).
Abstract
Hepatocellular Carcinoma (HCC) with Portal Vein Tumor Thrombus (PVTT) is a highly advanced unresectable tumor with limited treatment options and a rather poor prognosis. Recently, atezolizumab plus bevacizumab was introduced as a new first-line combination therapy regimen for unresectable-HCC (u-HCC), but its use is restricted to patients with compensated liver function (ie, Child-Pugh [CP] class A liver function). We describe a 68-year-old man with u-HCC with PVTT (Vp4) in the decompensated stage (CP-B; score 8), who was treated with atezolizumab plus bevacizumab followed by lenvatinib outside current clinical guidelines. At his first visit to our hospital, he was diagnosed with diffuse-type HCC in the S4 lobe of the liver and PVTT that spread to the main portal trunk (Vp4). Unfortunately, he was out of systemic chemotherapy due to his decompensated liver function. Despite his urgent condition, combination therapy was expected to provide a benefit. Indeed, his liver function improved to CP-A (score 6), and the primary HCC tumor was reduced in size, indicating partial efficacy of this regimen. However, after six cycles of combination therapy, progression of PVTT was observed on CT imaging. Following second-line treatment with lenvatinib, CP-A liver function was preserved, and no increase in hepatic tumors was observed, but PVTT had further progressed. After HCC brain metastasis caused left hemiplegia, acute bleeding of esophageal varices led to rapid deterioration of his general condition. His survival time of 8.5 months was slightly longer than that of reported high risk Vp4 cases. Due to limited treatment options and the poor prognosis of patients with Vp4 uHCC, this case can potentially be used to guide the possible treatment of patients with decompensated liver function outside current guidelines.
Keywords: Hepatocellular carcinoma; Atezolizumab plus bevacizumab; Lenvatinib; Portal vein tumor thrombus; Child-Pugh B.
Citation: Yoshikawa A, Fujimoto D, Miyagi A, Okushi S, Tanahashi T, et al. Atezolizumab plus bevacizumab followed by lenvatinib in a case of unresectable hepatocellular carcinoma with Vp4 and child-pugh B. J Gastroenterol Res Pract. 2023; 3(2): 1135.
Introduction
Hepatocellular Carcinoma (HCC) is the most common primary malignancy of the liver and the leading cause of cancer death worldwide [1]. Even when HCC is diagnosed at an early stage, repeat recurrences after curative treatment are common, and subsequent disease progression results in unresectable status. Unfortunately, this malignancy often presents as unresectable-HCC (u-HCC) at initial diagnosis. In addition, the prognosis of patients with advanced u-HCC with portal vein tumor thrombus (PVTT) is poor, and these patients have limited treatment options [2]. The main reason for the poor prognosis is impairment of liver function due to decreased portal blood flow caused by intra-hepatic metastasis with PVTT.
Current clinical guidelines for u-HCC recommend systemic chemotherapy with Molecular-Targeting Agents (MTA), including sorafenib and lenvatinib, since the initial approval of sorafenib in 2007. More recently, on the basis of the IMbrave 150 trial in 2020 [3], the combination of atezolizumab; a monoclonal antibody (mAb) directed against programmed cell death ligand 1 (PD-L1), and bevacizumab; a mAb directed against vascular endothelial growth factor (VEGF), represents a novel first-line chemotherapy regimen for patients with u-HCC [3,4]. Atezolizumab plus bevacizumab dramatically improves the prognosis of patients with advanced u-HCC, and this combination immunotherapy is significantly fewer toxicities compared with sorafenib [3]. However, the combination therapy in this trial was restricted to patients with preserved liver function (ie, Child-Pugh [CP] A disease) to reduce the incidence of adverse events (AEs).
Since an extensive number of novel therapeutic options are available for patients with advanced u-HCC, it is important to reevaluate patients with decompensated stage, who were not included in recent clinical trials. Indeed, initial evidence suggested that programmed cell death 1 (PD-1) monotherapy could be safely administered to patients with CP-B liver dysfunction [5-7]. In contrast, the AE profile of atezolizumab plus bevacizumab indicated immune-related pathology as well as a potential risk of bleeding, even in patients with preserved live function [3]. To date, no practical evidence has confirmed the safety and efficacy of atezolizumab plus bevacizumab in patients with u-HCC outside strict CP-A criteria.
Here, we report a patient of u-HCC with PVTT (Vp4) in the decompensated stage, who received atezolizumab plus bevacizumab followed by lenvatinib outside current practical guidelines.
Case presentation
A 68-year-old man was referred to our hospital from a local physician’s office for treatment of abdominal fullness. He was infected with the hepatitis B virus (HBV), and drank 1.5 cups/ liquor per day until the time of examination. Physical examination at the first visit to our hospital revealed no remarkable findings except for abdominal fullness. His height was 161.5 cm and body weight was 67.2 kg. Laboratory findings are shown in Table 1 and were as follows: white blood cells, 5.7 × 103/mm3 with normal differential; hemoglobin, 14.2 g/dL; platelets, 15.5 × 104/L; prothrombin activity (PT), 122%; C-reactive protein, 2.46 mg/dL; total protein, 7.1 g/dL; albumin, 3.6 g/dL; aspartate aminotransferase (AST), 159 U/L; alanine transaminase (ALT), 74 U/L; alkaline phosphatase, 630 U/L; lactate dehydrogenase, 707 U/L; total bilirubin, 3.5 mg/dL; alfa-fetoprotein (AFP), 643 ng/mL; des-gamma-carboxy prothrombin (DCP), 60,730 mAU/ mL; hepatitis B surface antigen, negative; hepatitis B surface antibody, positive; and Hepatitis C Virus (HCV) antibody, negative.
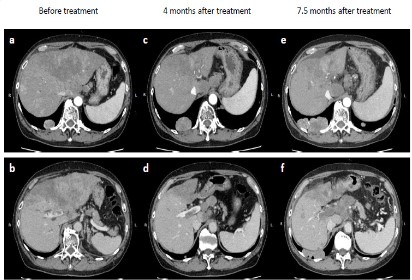
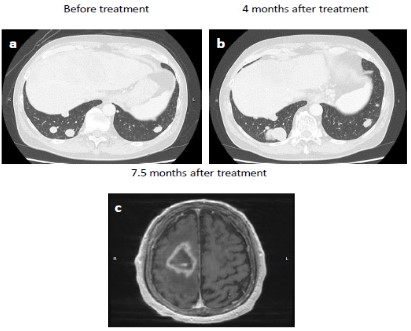
Abdominal enhanced Computed Tomography (CT) showed a large, irregularly shaped mass located primarily in segment 4 of the liver, as well as multiple smaller nodules (Figure 1a). CT scan further revealed PVTT that extended from the main portal vein to the right portal trunk (Figure 1b). Thoracic CT showed multiple nodules in both lungs, suggestive of extra-hepatic metastases (Figure 2a). Para-aortic and extrahepatic hilar lymph node swellings with ascites was also observed. Based on these radiological and laboratory findings, the patient was diagnosed with advanced diffuse-type HCC with PVTT (Vp4) in a background of chronic liver disease with HBV infection. Based on clinical staging, he was diagnosed with cT4N1M1 cStage IVB, CP-B (score, 8) HCC.
Clinical course
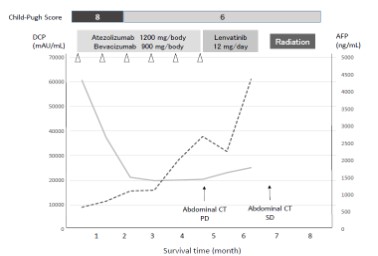
Although the patient was relatively young and had a good performance status score (0), current Japanese clinical guideline indicated that he was ineligible for systemic chemotherapy due to poor liver function (CP-B; score 8) [4]. However, we recommended atezolizumab (1,200 mg daily) plus bevacizumab (900 mg/body) as first-line treatment due to an expected improvement in liver function. Since no clinical trials for patients with CP-B were available, informed consent was fully obtained from the patient prior to treatment. After one cycle of this immunotherapy of combination regimen, his DCP level decreased to 37,437 mAU/mL. As expected, total bilirubin decreased to 0.9 mg/dL, and liver dysfunction improved to CP-A (score, 6). Considering this response to atezolizumab plus bevacizumab treatment, we recommended the patient to continue with this regimen.
After 4 months and six cycles of combination therapy, abdominal CT was conducted with the tumor assessment. Contrast-enhanced CT revealed size reduction of the primary liver tumor (Figure 1c). However, progression of PVTT was observed (Figure 1d). Despite lack of apparent ascites, lung metastases were increased in size (Figure 2b). In addition, serum DCP and AFP levels had also increased. According to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [8], the tumor response was considered to be Progressive Disease (PD).
At that time, we considered switching the patient to lenvatinib as second-line therapy. During the period of atezolizumab plus bevacizumab treatment, his performance status was maintained at 0, and he experienced no life-threatening AEs.
The patient started to take lenvatinib for 12 mg per day. After administration of lenvatinib, his liver function was maintained, indicated by CP-A liver function (score 6). At 3.5 month after initiation of lenvatinib, repeat CT assessment showed no significant increase in the size of hepatic tumors (Figure 1e), corresponding to Stable Disease (SD) by mRECIST. However, further progression of PVTT was observed (Figure 1f). At the same time, left hemiplegia suddenly occurred, and brain magnetic resonance imaging (MRI) indicated a metastatic tumor (Figure 2c). Therefore, lenvatinib administration was immediately stopped, and radiation therapy was initiated for brain metastasis. After the patient recovered from this brain metastasis, ruptured esophageal varices caused acute bleeding. Following emergency endoscopic treatment, his liver function and general condition deteriorated, and he died at 8.5 months after initiation of treatment. Clinical course is summarized in Figure 3.
a, c, e; arterial phase of enhancement. b, d, f; portal phase of enhancement.
Table 1: Laboratory data at the first visit to our hospital.
| Complete blood count | Blood chemistry | |||
|---|---|---|---|---|
| WBC | 5700 /uL | TP | 7.1 | g/dL |
| RBC | 397 x 104/uL | AlB | 3.6 | g/dL |
| Hemoglobin | 14.2 g/dl | T-Bil | 3.5 | mg/dL |
| Platelets | 15.5 x 104 /uL | AST | 159 | IU/L |
| ALT | 74 | IU/L | ||
| Coagulation test | ALP | 630 | IU/L | |
| PT (%) | 122 | Y-GTP | 1807 | IU/L |
| PT(sec) | 11.9 | LDH | 707 | IU/L |
| PT (INT) | 0.9 | BUN | 15 | mg/dL |
| APTT(INT) | 41.5 | Cre | 0.82 | mg/dL |
| Na | 140 | mEq/L | ||
| Hepatitis viral marker | K | 3.9 | mEq/L | |
| HBs-Ag | Negative | C1 | 8.8 | mEq/L |
| HBs-Ab | Positive | T-Cho | 217 | mg/dL |
| HBc-Ab | Positive | TG | 69 | mg/dL |
| HCV-Ab | Negative | NH3 | 86 | ug/dL |
| Tumor marker | CRP | 2.46 | mg/dL | |
| AFP | 643.8 ng/mL | |||
| DCP | 60730 mAU/mL | |||
WBC: white blood cell; RBC: red blood cell; PT: prothrombin time; APTT: activated partial thromboplastin time; HBs-Ag: hepatitis B surface antigen; HBs-Ab: hepatitis B surface antibody; HBc-Ab: hepatitis B core antibody; HCV-Ab: hepatitis C virus antibody; AFP: alpha fetoprotein; DCP: des-gamma-carboxyprothrombin; TP: total protein; Alb: albumin; T-Bil: total bilirubin; AST, aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; γ-GTP: gamma-glutamyltransferase; LDH: lactate dehydrogenase; BUN: blood urea nitrogen; Cre: creatinine; T-Cho: total cholesterol; TG: triglyceride; NH3: ammonia; CRP: C-reactive protein.
Discussion
We report a case of advanced u-HCC with CP-B liver function, in which atezolizumab plus bevacizumab followed by lenvatinib therapy resulted in partial regression of the primary hepatic tumor and contributed to improve the prognosis of the patient.
HCC is often diagnosed at advanced stages with limited curative therapy options, leading to a 5-year survival rate of 2% [1]. The current (2022; fourth edition) clinical practice guideline of the Japan Society of Hepatology for advanced u-HCC strongly recommend the combination of atezolizumab plus be vacizumab as first-line therapy [4]. However, this recommendation is strictly intended for CP-A cases. The majority of systemic therapies for HCC have been studied in CP-A populations, which means that preserved liver function is favorable for registration into clinical trials. Although patients with HCC with CP-B cirrhosis represent a growing population with a poor prognosis, such patients have limited treatment options [9]. In addition, no prospective studies have been performed in patients in the decompensated stage to date. The present case was diagnosed with advanced uHCC with PVTT at his first visit to our hospital, indicating the liver dysfunction of CP-B. While not included in the guidelines for such a patient, atezolizumab plus bevacizumab treatment resulted in a temporary reduction in the size of the primary tumor, continuous improvement of liver dysfunction, and a decreased DCP level, suggesting the feasibility of this combination regimen for patients with uHCC in association with decompensated cirrhosis. However, future retrospective and prospective clinical trials are required to fully establish the safety and efficacy of atezolizumab plus bevacizumab in patients with advanced HCC with CP-B live function.
The prognosis of u-HCC with PVTT is rather poor with estimated survival of 2-4 months in the absence of treatment [2, 10]. In particular, Vp4 (portal invasion at the main portal trunk) should be considered as a clinical emergency that can become fatal within just 2 weeks [11]. The efficacy of sorafenib for PVTT was limited with reported median survival for patients with HCC and Vp3/4 PVTT of only 3-4 months [12]. With the development of lenvatinib and atezolizumab plus bevacizumab, systemic chemotherapeutic strategy for u-HCC has completely changed [2, 3]. The efficacy of the combination therapy was demonstrated in the IMbrave150 trial, with objective response and disease control rates of 33.2% and 72.3%, respectively [3]. However, this trial included high-risk cases, defined as patients with Vp4 portal vein thrombus, bile duct invasion, or liver infiltration >50% [13]. Subgroup analyses revealed that median overall survival among highrisk cases was only 7.6 months, compared with 22.8 months in no high-risk cases [13]. In the present high-risk case with Vp4 PVTT, survival time after initiation of combination therapy was 8.5 months, which is slightly longer than previously reported.
After failure of atezolizumab plus bevacizumab, the efficacy of lenvatinib has been reported as second-line or sequential therapy [14]. PD-1 and PD-L1 mAbs are expected to remain bound to their antigens on T cells and cancer cells, continuously for a certain period, and further treatment with lenvatinib, a multikinase inhibitor, may have a synergistic role, such as inhibition of neoangiogenic and immunosuppressive effects in the tumor microenvironments [15]. In the present case of HCC associated with HBV, the efficacy of lenvatinib was insufficient. Recently, the different efficacy of MTA has been of particular interest with focus on the different etiologies underlying the pathogenesis of liver cancer. In fact, viral and/or non-viral etiologies might have effect on the immune context of HCC and thus differential responses to treatment [16]. More recently, lenvatinib treatment was shown to be associated with a significant survival benefit in nonviral HCC patients compared with atezolizumab plus bevacizumab [17]. However, additional studies are needed to clarify the differential efficacy of lenvatinib among liver cancers of different etiologies.
In the IMbrave 150 trial [3], bleeding events were more frequently observed with atezolizumab plus bevacizumab than with sorafenib (25.2% versus 17.3%, respectively), including gastrointestinal bleeding (7% versus 4.5%, respectively) [3]. Gastrointestinal bleeding is a well-known AE associated with bevacizumab, and life threatening complication among patients with HCC. More recently, rapid progression of esophageal varices was reported after treatment with atezolizumab plus bevacizumab [18]. In addition, a rare case of liver fibrosis and sinusoidal obstruction was reported after treatment with atezolizumab [19]. Although bevacizumab is a mAb directed against VEGF, which plays a major role in portal hypertension, the exact effects of atezolizumab plus bevacizumab on progression of portal hypertension remain controversial. In the present case, in addition to AEs associated with immunotherapy-based combination, persistent growth of PVTT could have contributed to bleeding event via portal hypertension. To ensure safety during administration of atezolizumab plus bevacizuma, great caution is required, as bleeding complications are a direct cause of deterioration of the general condition of HCC patients in the decompensated stage.
Conclusion
We have presented a case of u-HCC with Vp4 PVTT and CP-B liver function treated with atezolizumab plus bevacizumab followed by lenvatinib, which resulted in partial tumor regression, and an improved poor prognosis. Given the limited therapeutic options and low survival for patients with Vp4 u-HCC, this case suggest a possible treatment for patients in the decompensated stage outside current guidelines.
Declarations
Acknowledgements: Not applicable.
Conflict of interest: All authors declare that they have no competing interests and funding support.
Human rights: All procedures were performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Informed consent: Informed consent was obtained from the patient.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. Ca Cancer JClin. 2022; 72: 7-33.
- Vauthey JN, Lauwers GY, Esnaola NF, Jacques B, Nadeem M, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002; 20: 1527-1536.
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382: 1894-1905.
- Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021; 10: 181-223.
- Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, et al. Check-Mate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021; 75: 600-609.
- Fessas P, Kaseb A, Wang Y, Saeed A, Szafron D, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020; 8: e001033.
- Kambhampati S, Bauer KE, Bracci PM, Keenan BP, Behr SC, et al. Nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh class B cirrhosis: safety and clinical outcomes in a retrospective case series. Cancer. 2019; 125: 3234-3241.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30: 52-60.
- Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017; 18: e101-12.
- Quirk M, Kim YH, Saab S, Lee EW. Management of hepatocellular carcinoma with portal vein thrombosis. World J Gastroenterol. 2015; 21: 3462-3471.
- Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011; 29: 339-364.
- Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, et al. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer. 2017; 6: 275-286.
- AL Cheng, S Qin, M Ikeda, Galle PR, Ducreux M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. Journal of Hepatology. 2022; 76: 862-863.
- Aoki T, Kudo M, Ueshima K, Morita M, Chishina H, et al. Exploratory analysis of lenvatinib therapy in patients with unresectable hepatocellular carcinoma who have failed prior PD-1/PD-L1 checkpoint blockade. Cancers (Basel). 2020; 12: 3048.
- Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020; 38: 2960-2970.
- Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, et al. NASH limits antitumour surveillance in immunotherapy-treated HCC. Nature. 2021; 592: 450-456.
- Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022; 7: 100591.
- Furusawa A, Naganuma A, Suzuki Y, Hoshino T, Yasuoka H, et al. Two cases of rapid progression of esophageal varices after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Clin J Gastroenterol. 2022; 15: 451-459.
- Honma Y, Shibata M, Gohda T, Matsumiya H, Kumamoto K, et al. Rapid progression of liver fibrosis induced by acute liver injury due to immune-related adverse events of atezolizumab. Intern Med. 2021; 60: 1847-1853.