Journal of Gastroenterology Research and Practice
Research Article - Open Access, Volume 3
Perioperative chemotherapy versus upfront surgery with adjuvant chemotherapy in gastric and esophagogastric junction adenocarcinoma: A retrospective trial
Veruska Peron1; Alan Felcar Soares1; Ana Maria Ulbritch Gomes1; Rivadávio AM de Oliveira1; Caio C dos Santos Kasai2; Everton GA Melo1*
1Department of Clinical Oncology, Londrina Cancer Hospital, PR, Brazil.
2Economist by Faculty of Economics and Administration at São Paulo University (USP), São Paulo, SP, Brazil.
*Corresponding Author : Everton G A Melo
Department of Clinical Oncology, Londrina Cancer
Hospital, PR, Brazil.
Email: evertongermano@yahoo.com.br &
evertongermanomelo@gmail.com
Received : Feb 12, 2023
Accepted : Mar 13, 2023
Published : Mar 22, 2023
Archived : www.jjgastro.com
Copyright : © Melo EGA (2023).
Abstract
Objective: Gastric cancer represents a common cause of cancer death worldwide. Adenocarcinoma accounts for more than 95% of all gastric malignancies. Multimodal approaches in the treatment of localized gastric cancer have changed the natural history of the disease. Compared with surgery alone, several therapeutic approaches including perioperative chemotherapy or adjuvant chemotherapy improve survival. This study aimed to compare perioperative chemotherapy and upfront surgery with adjuvant chemotherapy in gastric and esophagogastric junction adenocarcinoma, in terms of clinical features, survival outcomes and prognostic factors.
Methods: Retrospective, single-center study, whose data were collected from electronic medical records by three investigators. Patients aged 18 years or older, treated in the public health system, between January 1st, 2015 and December 31st, 2020, diagnosed with gastric or esophagogastric junction adenocarcinoma Siewert 2 or 3, with cT2-4 or cN + (M0) staging were included. OS and PFS curves were developed according to the Kaplan-Meier method and they were compared using the log-rank test.
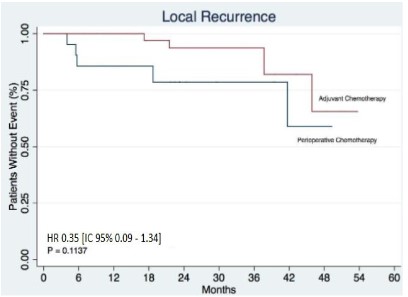
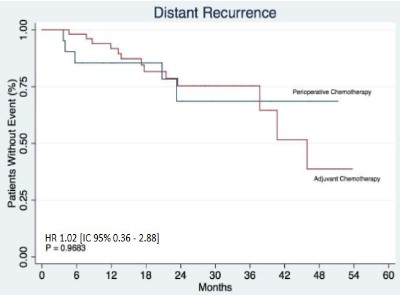
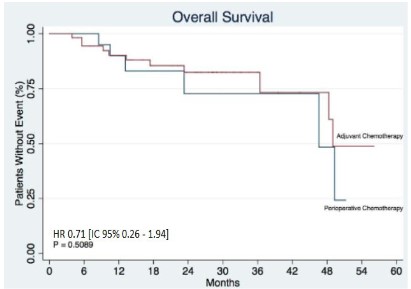
Results: In terms of locoregional and distant recurrence, no statistically significant differences were found between the groups, HR 0.35 [IC 95% 0.09-1.34 p:0.1137] and HR 1.02 [IC 95% 0.36–2.88 p:0,9683], respectively. In the analysis of overall survival, no statistically significant difference was observed, HR 0.71 [IC 95% 0.26–1.94 p: 0.5089].
Conclusion: In this study, statistically significant differences were not observed for PFS and OS in the treatment of patients with localized gastric cancer regarding the use of perioperative chemotherapy versus upfront surgery with adjuvant chemotherapy. In the last decade, there has been a drive towards improving perioperative treatment strategies. Future directions with the incorporation of targeted therapy, immunotherapy, and ctDNA analysis will likely consolidate the perioperative treatment strategy as standard.
Keywords: Gastric cancer; Esophagogastric cancer; Perioperative chemotherapy; Adjuvant chemotherapy.
Citation: Peron V, Soares AF, Gomes AMU, de Oliveira RAM, Melo EGA, et al. Perioperative chemotherapy versus upfront surgery with adjuvant chemotherapy in gastric and esophagogastric junction adenocarcinoma: A retrospective trial. J Gastroenterol Res Pract. 2023; 3(2): 1132.
Introduction
Gastric cancer represents a common cause of cancer death worldwide [1]. More than 26,000 new cases are estimated in United States (US) 2021 with 11,000 deaths [2]. In Brazil it represents the 4th most incident cancer in men and the sixth in women, being responsible for more than 15,000 deaths [3]. Risk factors for gastric cancer include variables, such as age, sex, and race/ethnicity, infection with Helicobacter pylori bacteria, smoking, and diets high in nitrates and nitrites [4].
Adenocarcinoma accounts for more than 95% of all gastric malignancies [5]. According to the Lauren classification, gastric cancer can be subdivided into intestinal and diffuse types [6]. With molecular analysis of TCGA, these tumors were first categorized by EBV-positivity (9%), MSI status (22%), genomically stable (20%) or those exhibiting chromosomal instability (CIN; 50%). These molecular characteristics provides distinct subtypes of gastric cancer facilitating the development of clinical trials to explore therapies in defined sets of patients, ultimately improving survival from this deadly disease [7].
Multimodal approaches in the treatment of localized gastric cancer have changed the natural history of the disease. Compared with surgery alone, several therapeutic approaches including perioperative chemotherapy or adjuvant chemotherapy improve survival [8]. On the other hand, there is no phase 3 study comparing the strategy of perioperative chemotherapy versus upfront surgery and adjuvant chemotherapy in the setting of localized gastric cancer disease.
This study aimed to compare perioperative chemotherapy and upfront surgery with adjuvant chemotherapy in gastric and esophagogastric junction adenocarcinoma, in terms of clinical features, survival outcomes and prognostic factors of patients treated in Londrina Cancer hospital by the public health system.
Patients and methods
Patient Population
Patients aged 18 years or older, treated in the public health system, between January 1st, 2015 and December 31st, 2020, diagnosed with gastric or esophagogastric junction adenocarcinoma Siewert 2 or 3, with cT2-4 or cN+ (M0) staging were included. Patients with secondary malignancy, insufficient data on electronic medical record, metastatic disease at diagnosis and patients not undergoing surgical treatment at our institution were excluded from the study.
Data collection and statistical analysis
This was a retrospective, single-center study, whose data were collected from electronic medical records by three investigators. TASY system was used, and the medical records were screened by searching for CIDs C15 and C16. In addition, the databases were revised for patients undergoing esophagus-gastrectomy at our institution in the period outlined by the study.
Demographic characteristics were assessed through descriptive analyzes using frequencies and medians. Association analyzes between categorical variables were performed using the Chi-square test or Fisher’s exact test to assess characteristics between the groups. Overall survival (OS) was defined as the time between the beginning of treatment and death or last follow-up and distant or locoregional progression free survival (PFS) as the time between the beginning of treatment and the progression of disease or death. OS and PFS curves were developed according to the Kaplan-Meier method and they were compared using the log-rank test. A p value lower than 0.05 was considered statistically significant. The program used was the statistical software STATA® version 17.0.
Results
Between January 1st, 2015 and December 31st, 2020, 76 patients treated at the Londrina Cancer Hospital were retrospectively evaluated. Baseline characteristics of the 2 groups are de- scribed in Table 1. Of these patients, 21 underwent a treatment strategy with perioperative chemotherapy. The male population of this group represented 57%, with a median age of 66 years old, more than 90% of patients were ECOG 0 or 1 and 67% had gastric cancer. Regarding clinical staging, 87% were stage 3. Of the 21 patients treated with perioperative chemotherapy, 15 underwent chemotherapy with FOLFOX and 6 used XELOX (Table 2). The other group was represented by 55 patients, who underwent upfront surgery. Of these, 60% of them were men with a mean age of 64 years. More than 90% of them were ECOG 0 or 1. In this group, 96% had gastric cancer and 54% were clinical stage 3. In this adjuvant setting, 45 patients were treated with XELOX while only 9 patients used FOLFOX. Only 1 patient required a switch from XELOX to FOLFOX treatment due to intolerance (Table 3).
When the resection margin was evaluated, it was observed that 93% of the patients undergoing the perioperative treatment strategy had a R0 resection rate, on the other hand, this rate was 78% in the group of patients undergoing upfront surgery. In addition, 31% of patients in the first group had a complete pathological response. Regarding surgical morbidity, there as 1 death in the group of patients undergoing upfront surgery.
In terms of locoregional and distant recurrence, no statistically significant differences were found between the groups, HR 0.35 [IC 95% 0.09-1.34 p:0.1137] and HR 1.02 [IC 95% 0.36-2.88 p:0.9683], respectively (Figures 1 and 2). In all, 9 (11.84%) local recurrences were observed in a total of 76 patients. Note that it was not possible to compute the median locoregional disease-free survival time, since not even 50% of the patients had this event. In the perioperative chemotherapy group, 5 (26.32%) of the 19 patients (note that there were 2 pieces of information here) had local recurrence, while in the adjuvant chemotherapy group, 4 (7.27%) of the 55 patients had this event. In terms of distant disease- free survival, it was observed that a total of 18 (23.68%) of the 76 patients had this event. The median follow-up period was 21.4 (months). It was possible to observe that 5 (26.32%) of the 19 patients who received perioperative chemotherapy (2 missing data) had distant recurrence, while this fact occurred in 13 (23.64%) of the 55 patients who underwent upfront surgery.
Finally, in the analysis of overall survival, it was observed a total of 76 individuals who were followed up for an average time of 22.6 (months).
During this period, 17 events were observed, corresponding to a total of 22.37%. Considering the treatment groups, it was observed that in patients who received perioperative chemotherapy, 6 events (28.57%) were recorded in this period. The median survival for this group was 46.6 months. On the other hand, in patients treated with adjuvant chemotherapy, 11 events (20%) were observed.
The median survival of this group was 49 months. In this sense, it is possible to state that there is no statistically significant difference for overall survival, HR 0.71 [IC 95% 0.26–1.94 p:0.5089] (Figure 3).
Table 1: Descriptive Analysis.
Characteristics - Group |
pCT |
aCT |
pCT/aCT |
21 (27.6%) |
55 (72.3%) |
Age |
||
|---|---|---|
Median |
66years (59-69) |
64 years(53-70) |
Sex |
||
Male |
12 (57%) |
33(60%) |
Female |
9 (43%) |
22 (40%) |
ECOG |
||
0-1 |
20(95.3%) |
53(96%) |
02-Mar |
1 (4.7%) |
2 (4%) |
Grade |
||
G1 |
2(11%) |
5(9,6%) |
G2 |
13 (68%) |
19 (36.5%) |
G3 |
4 (21%) |
28 (53.8%) |
Loaction |
||
Siewert 2 |
3(14%) |
0 (0%) |
Siewert 3 |
4 (19%) |
2 (3.8%) |
Gastric |
14 (67%) |
53 (96.2%) |
Clinical Staging |
||
l |
0 (0%) |
3(12.5%) |
ll |
2 (13%) |
8 (33.3%) |
llI |
13 (87%) |
13 (54.1%) |
Resection Margin |
||
R0 |
14(93.3%) |
43(78.1%) |
R1 |
1 (6.7%) |
7 (12.7%) |
R2 |
0 (0%) |
5 (9.2%) |
Table 2: Perioperative chemotherapy protocols (pCT).
aCT |
Total 55 (100%) |
|---|---|
FOLFOX |
9 (16.4%) |
XELOX |
45 (81.8%) |
Change of treatment |
1 (1.8%) |
Table 3: Locoregional and distant recurrence.
Disease relapse |
pCT |
aCT |
|---|---|---|
Locoregional |
5 (26%) |
4 (7%) |
Distant |
5 (26%) |
13 (24%) |
Discussion
The multimodal approach for the treatment of gastric and gastroesophageal adenocarcinoma Siewert 2 or 3 is the strategy that has been consolidated so far. In this context, perioperative chemotherapy and surgery followed by adjuvant chemotherapy are two possible strategies
In this study there was no statistically significant difference in OS and PFS between the groups treated with perioperative chemotherapy or upfront surgery followed by adjuvant chemotherapy. It is important to note that perioperative chemotherapy protocol with XELOX or FOLFOX is not the standard treatment in this context. The FLOT4 trial demonstrated the superiority of the FLOT regimen when compared directly with ECF / ECX. In that trial, 716 patients with gastric or gastroesophageal junction adenocarcinoma (56% of patients) with tumors ≥cT2 and / or N+ were randomized to 4 cycles of FLOT followed by surgery followed by 4 cycles of FLOT versus 3 cycles of ECF / ECX followed by surgery followed by 3 ECF / ECX cycles. With a median follow-up of 43 months, FLOT resulted in an increase in OS (median of 35 versus 50 months, HR = 0.77; 95% CI: 0.63-0.94; p= 0.012), PFS (median of 18 versus 30 months, HR = 0.75; 95% CI: 0.62-0.91; p=0.004) and pCR (16 versus 6%; p=0.02) [8].
Unfortunately, the FLOT protocol is not available in all the Brazilian centers for the treatment of patients in the public health system. Based on the robust results of this study, perioperative chemotherapy strategies have been consolidated. In a phase III trial evaluating FOLFOX and a cisplatin-fluorouracil regimen in metastatic gastroesophageal adenocarcinoma, FOLFOX was better tolerated with less toxicity than the cisplatin-based protocol and was more effective in older patients [9]. Then, the FOLFOX-based perioperative regimen achieves favorable results in real life practice. The optimal number of chemotherapy cycle remains to be determined. There are great future prospects for treatment in this context for which data are expected, such as the KEYNOTE-585 phase 3 study that evaluates the efficacy of pembrolizumab associated with a perioperative chemotherapy strategy for the treatment of gastric and gastroesophageal junction adenocarcinoma [10].
Considering the patients who underwent neoadjuvant chemotherapy in this study, 93% had R0 surgery, whereas in patients operated on upfront, the rate of R0 surgery was 78%. The presence of R0 surgery correlates with a better prognosis [11], however, due to the low number of patients in this study, it was not possible to observe this correlation.
On the other hand, there are still many patients who undergo upfront surgery before conducting a multidisciplinary discussion. In these cases, the adjuvant treatment strategies should be discussed. In the scenarios of patients undergoing adequate surgery with D2 lymphadenectomy, the ARTIST study evaluated the role of postoperative adjuvant QT and RT in 458 patients comparing capecitabine and cisplatin (XP) for 6 cycles versus 2 cycles of pre XP and post 45 Gy of RT with capecitabine 1650 mg/m²/day. This study did not demonstrate an advantage of disease free survival (DFS) for the arm including RT, but in sub-group analysis there was an advantage of DFS for the RT arm in individuals with positive lymph nodes [12]. The ARTIST II study specifically compared the hypothesis of adding adjuvant RT to QT in individuals with type D2 resection and positive lymph node, as well as comparing adjuvant QT with S-1 versus S-1 combined with oxaliplatin, showing that RT does not bring addition of benefit over S-1 combined with oxaliplatin and that both strategies are superior to the use of S-1 alone [13]. In the con- text of patients undergoing upfront surgery with D2 lymphadenectomy, it is possible to consider adjuvant chemotherapy with the association of platinum and fluoropyrimidine. The CLASSIC study, with 1035 patients, which evaluated adjuvant XELOX versus observation after type D2 resection, demonstrated an advantage in DFS at 3 years (74 versus 59%; HR=0.56; p<0.0001) and a trend towards improvement in OS at 3 years (HR=0.72; p=0.0493) [14]. An update of this study demonstrated a decrease in the risk of death from 27 to 20% (HR=0.66; 95% CI: 0.51-0.85; p=0.0015), confirming the benefit of adjuvant XELOX as well in terms of OS [15].
The standard treatment for locally advanced gastric cancer differs across the world. In western countries, perioperative chemotherapy or postoperative adjuvant chemoradiotherapy are the preferred treatment options, whereas in Asia, D2 gastrectomy followed by postoperative adjuvant chemotherapy is standard [16].
For patients with HER2-positive gastric cancer, the addition of trastuzumab to perioperative cytotoxic chemotherapy is a plausible treatment option to improve survival outcomes [17]. The randomized phase II PETRARCA study, presented at ASCO 2020, evaluated perioperative CT with FLOT in combination with trastuzumab and pertuzumab versus perioperative CT with FLOT. In that study, the use of perioperative anti-HER-2 therapy resulted in a higher pCR rate (35 versus 12%; p=0.02), but preliminary data did not demonstrate gains in DFS and OS [18]. Therefore, so far there is no con- sistent evidence for the use of this association.
The limitations of the study are due to a retrospective character, performed in a single institution and because it has a small number of cases. There is no definitive trial that has compared perioperative chemotherapy versus upfront surgery followed by adjuvant chemotherapy for the treatment of gastric and gastroesophageal cancers. In the last decade, there has been a drive towards improving perioperative treatment strategies. Future directions with the incorporation of targeted therapy, immunotherapy, and ctDNA analysis will likely consolidate the perioperative treatment strategy as standard.
Conflicts of interest: All authors declare that they have no conflicts of interest regarding the content of this research.
References
- World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2021: Estimated Cancer Incidence, Mortality and Prevalence Worldwide.
- The American Cancer Society. Cancer Statistics Center.
- National Cancer Institute. Cancer Statistics.
- American Institute for Cancer Research, World Cancer Research Fund. Stomach Cancer. How Diet, Nutrition, and Physical Activity Affect Stomach Cancer Risk.
- Shiliang Ai, Chen Li, Xiaoyan Li, Jiang T, Grzegorzek M, et al. A State-of-the-Art Review for Gastric Histopathology Image Analysis Approaches and Future Development. BioMed Research International. 2021; 2021: 6671417.
- Laurén, P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal- type carcinoma. Acta Pathol Microbiol Scand. 1965; 64: 31-49.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513: 202-209.
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019; 393: 1948-1957.
- Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, et al. Phase III Trial in Metastatic Gastroesophageal Adenocarcinoma with Fluorouracil, Leucovorin Plus Either Oxaliplatin or Cisplatin: A Study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008; 26: 143542.
- Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, et al. KEYNOTE- 585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019; 15: 943-952.
- Biondi A, Persiani R, Cananzi F, Zoccali M, Vigorita V, et al. R0 resection in the treatment of gastric cancer: room for improvement. World journal of gastroenterology. 2010; 16: 3358-3370.
- Lee J, Lim DH, Kim S, Park SH, Park JO, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: The ARTIST trial. J Clin Oncol. 2012; 30: 268.
- Park SH, Zang DY, Han B, Ji JH, Kim TG, et al. ARTIST 2: interim results of a phase III trial involving adjuvant chemotherapy and/or chemora-diotherapy after D2-gastrectomy in stage II/III gastric cancer (GC). J Clin Oncol. 2019; 37: 4001.
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised con- trolled trial. Lancet. 2012; 379: 315-321.
- Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15: 1389-1396.
- Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today. 2020; 50: 30-37.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open label, randomised controlled trial. Lancet. 2010; 376: 687-697.
- Hofheinz RD, Haag GM, Ettrich TJ, Borchert K, Kretzschmar A, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarci- noma: final results of the PETRARCA multicenter randomized phase II trial of the AIO. J Clin Oncol. 2020; 38: 4502.