Journal of Gastroenterology Research and Practice
Research Article - Open Access, Volume 3
Low protein intake in postpartum women is associated with higher risk of gallbladder stasis and gallstone
Kuldip Kumar Solanki1; Alok Kumar1; Jimil Shah1; Rashmi Bagga2; Behera BN1; Shanti Devi1; Reena Das3; Birender Nagi1; Roshan Agarwala1; Rakesh Kochhar1; Usha Dutta1*
1Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
2Department of Obstetrics & Gynecology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
3Department of Hematology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
*Corresponding Author : Usha Dutta
Department of Gastroenterology, Postgraduate
Institute of Medical Education and Research,
Chandigarh, India.
Tel: +91-172-2756610; +91-8198877022;
Email: ushadutta@gmail.com
Received : Feb 06, 2023
Accepted : Mar 10, 2023
Published : Mar 17, 2023
Archived : www.jjgastro.com
Copyright : © Dutta U (2023).
Abstract
Background and Aim: Gallstones (GS) are traditionally associated with “fat fertile forty females”. However, in India, GS are often found in young malnourished multiparous women. We conducted this study to determine the influence of nutritional inadequacy on prevalence of gallbladder (GB) stasis and GS among postpartum women.
Methods: A prospective cross-sectional study was conducted in a tertiary care center. Women after normal term delivery were evaluated at 6 weeks postpartum. Detailed clinical evaluation, anthropometric measurements, dietary profile and laboratory tests were done. Ultrasound (USG) was done to assess the presence of GS, sludge and ejection fraction (GBEF) after a standard fatty meal. Female volunteers (n=22) of similar age group were assessed by the same protocol.
Results: The mean age of cases (n=81) was 25.6 ± 3 years. Mean fasting GB volume, GBEF and residual GB volume at 60 mins was significantly higher amongst postpartum women (PPW) compared to volunteers (p<0.001). PPW with GB stasis had lower BMI (21.4 ± 3.7 vs 23.8 ± 3.6; p=0.013) and consumed significantly lesser protein intake (58 ± 10 vs 69.4 ± 16.8; p<0.001) compared to patients without stasis. Lower BMI & lower calorie intake were independent predictors of lower GBEF. PPW with GS (n=8) had more often use of protein deficient diet(100% vs 71%; γ=0.004), were more often multiparous (75% vs 22%; p=0.001) and had lower serum ferritin levels (20.3 ± 10.5 vs 41.7 ± 38 μg/L; p=0.001).
Conclusion: Protein deficient diet, low serum ferritin and poor nutritional status were associated with increase prevalence of GB stasis and GS among postpartum women. Lower BMI and lower calorie intake were independent predictors of lower GBEF.
Keywords: Protein deficiency; Iron deficiency; Gallstones; Post-partum period; Gall bladder ejection fraction.
Citation: Solanki KK, Kumar A, Shah J, Bagga R, Dutta U, et al. Low protein intake in postpartum women is associated with higher risk of gallbladder stasis and gallstone. J Gastroenterol Res Pract. 2023; 3(1): 1131.
Introduction
Cholelithiasis is a common health problem and affects about 10% of the western population and about 5% of the population in developing countries like India [1]. Gallstone (GS) disease is associated with significant morbidity and several life-threatening complications including gall bladder carcinoma. Gallstones has been traditionally said to be more common among the fat, fertile females in their forties. However, in India we find that a significant number of our patients with gallstones are lean, nutritionally deficient, multi-parous young women of low socio-economic status. Elucidating the risk factors for gallstones in these patients would provide an insight into the understanding of the pathogenesis of gallstone disease and thus aid development of preventive strategies.
Gallstones (GS) are approximately twice more common in females as compared to males and women develop GS at a younger age than men. Parity is an important risk factor for GS and most GS develop during pregnancy or in the immediate postpartum period [2,3]. Pregnancy is associated with combination of increased cholesterol saturation in the bile and gallbladder stasis largely attributed to the hormonal changes and dietary excesses [3,4]. However, pregnancy is associated with increased nutritional requirements, which if inappropriately met may result in deficiency states. In India, as there is significant gender inequality in economic and social spheres, results in significant malnutrition among women.
Iron deficiency has been found to enhance cholesterol GS formation. When adult prairie dogs were fed on iron deficient diet for 8 weeks, 80% developed cholesterol monohydrate crystals in their bile and 30% developed GS [5]. Iron deficiency reduces the activity of 7-hydroxylase and diminishes the gall-bladder (GB) neuronal nitric oxide synthase activity, thus contributing to cholesterol supersaturation of bile and GB stasis respectively6. Hypercholesterolemia and diet high in fat are also associated with increased risk for GS [6,7].
The interplay of these factors and diet in development of GS has not been studied in post-partum women. Hence, we planned this study to assess the prevalence and risk factors of GB stasis, GB sludge and GS in the post-partum women.
Methods
A prospective study was conducted at a tertiary care referral center in North India from 2004 to 2008. The study was conducted in full compliance with the guidelines of Good Clinical Practice and the Helsinki declaration. It was approved by the Ethics Committee of the Institute. All patients provided written informed consent before participating in the study. Postpartum women at six weeks after delivery of a single live baby after 36 weeks of gestation attending the postpartum clinic, were included as cases. Patients with history of prior cholecystectomy, recurrent pancreatitis, biliary colic, use of drugs (calcium channel blockers, erythromycin, prokinetics) in the preceding two weeks, non-availability of consent and history of complications during pregnancy like intrauterine growth retardation, congenital malformations, intrauterine deaths, still births, multiple gestations were excluded. Healthy young women volunteers (n=22) without GS or sludge on ultrasound were evaluated for GB function after a standard fatty meal to provide normative data.
The demographic factors, occupation, religion, socio-economic status, parity, outcomes of present and previous pregnancies, weight gain during pregnancy were obtained and recorded in a proforma. A detailed nutritional assessment was done by a trained dietician by the food frequency questionnaire in all patients [8]. Optimal cut-off for fat intake (up to 30 gm/ day in pregnancy and up to 45 gm/day in post-partum state) and protein intake (>65 gm/day in pregnancy and >75 gm/day in post-partum period) was considered as per recommendation [9]. Presence of biliary colic, jaundice, gallstones and cholestasis during pregnancy was recorded. Detailed physical examination including height, weight, body mass index, waist circumference, hip circumference, waist/hip ratio, triceps and biceps fold thickness, mid arm circumference was assessed. All patients underwent laboratory investigations including hemogram, liver function test, lipid profile and serum iron studies.
GB function was assessed using real time ultrasound examination by a single GI radiologist (BN) after an overnight fast for presence of sludge and gallstones. GB volume was measured using ellipsoid method as described by Dodd’s et al: Volume = 0.52 x L x W x H, where L is the length, W is the width and H is the height of GB [10]. All the dimensions were taken as the mean of three consecutive measurements. GB emptying was studied in response to a standard fatty meal. The meal consisted of 50 gms of fat, 20 gms of protein and 50 gms of carbohydrate. Gall bladder images were obtained at 30, 45 and 60 minutes after a fatty meal. The basal volume (ml), ejection fraction at 30, 45 and 60 minutes, time at peak contraction was calculated. Those with GBEF of <40%, they were classified as static GB [11]. Those in whom time to achieve maximum ejection was beyond 45 minutes after a meal were classified as sluggish GB. Female healthy volunteers of similar age group were assessed for GB function using ultrasound to understand the pattern of normal GB contraction.
Statistical analysis
Data was analyzed in SPSS version 17. GB emptying characteristics were compared between cases and controls. Univariate analysis was done to assess factors which are associated with gall bladder hypomotility, gallbladder sludge and gallstones. Chi-square test was used for categorical data and Student ‘t’ test was used for continuous data. Fisher’s exact test, Paired t test and Mann Whitney U test was used wherever appropriate. Gamma Statistics was used for ordinal variables. A p value of <0.05 was taken as significant.
Results
The mean age of cases (n=81) and controls (n=22) was 25.6 ± 3 years and 22.3 ± 6.1 years respectively (p>0.05). Most of the cases were primiparas (73%; 59 patients). Mean BMI of our postpartum patients was 23.98 ± 4.1 with 18 patients were overweight (BMI>25), 3 patients obese (BMI>30) and 2 patients underweight and rest patients (n=58) having BMI in normal range. Twelve cases had pregnancy associated hypertension and none had gestational diabetes. The most common biliary symptom during pregnancy was biliary colic which was found in 8 (10%) cases. Cholestasis of pregnancy was present in five (6.2%) cases (all had pruritus and one had jaundice). Positive family history of gallstones was found in 3 cases.
GB ejection fraction
The GBEF was significantly lower among cases compared to controls (54.5 ± 17 vs 63.4 ± 8.5; p<0.001). Mean fasting volume was significantly higher in cases compared to the controls (21.88 ±10.7 ml vs 16.5 ± 5.2 ml; p=0.002). The residual gallbladder volume at 60 minutes was also higher among cases than controls (11.2 ± 6.4 ml vs 5.8 ± 1.3 ml; p<0.001). GB stasis was present more often among cases than controls (25% vs 0%; p=0.009). Cases were more often had sluggish GB than controls (29.63% vs 4.5%; p=0.015) (Table 1).
Factors associated with GB Stasis
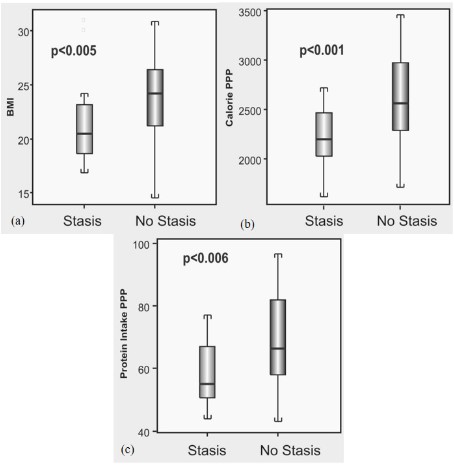
Patients with GB stasis had lower mean BMI (21.4 ± 3.7 vs 23.8 ± 3.6; p=0.013) and lower mean body weight (50.8 ± 11.5 kg vs 57.9 ± 8.5 kg; p=0.004) than those without stasis. However, patients with GB stasis had no significant difference in height compared to those without stasis (153 ± 7.7 cm vs 156 ± 6.6 cm; p=0.141). Patients with GB stasis had lower mean waist circumference and lower mean hip circumference than those without stasis (79 ± 13 cm vs 86 ± 9.7 cm; p=0.014) and (90 ± 9 cm vs 96 ± 7 cm; p=0.003) respectively. There was no difference in the waist hip ratio between the two groups (0.875 ± 0.06 vs 0.896 ± 0.06; p=0.20). Patients with GB stasis had lower mean triceps and biceps fold thickness than those without stasis (18 ± 5 mm vs 21 ± 5.3 mm; p=0.035) and (7.7 ± 2.5 mm vs 10.3 ± 4.1 mm; p=0.001) respectively.
Patients with GB stasis had lower mean daily calorie intake
compared to those without GB stasis (2237 ± 289 vs 2636 ± 521 Kcal; p<0.001). Patients with GB stasis had lower mean daily fat intake (84.9 ± 20 gm vs 104.6 ± 33 gm; p=0.015) and lower mean daily protein intake (58 ± 10 gm vs 69.4 ± 16.8 gm; p<0.001) (Table 2) (Figure 1). Patients with GB stasis had no significant difference in the iron profile and lipid profile. Patients with stasis more often had clinical signs of malnutrition such as Knuckle hyper pigmentation (54% vs 15%, p=0.008), cheilitis (91% vs 35%; p=0.001), frequent cramping in the legs (100% vs 44%; p=0.001) and proximal muscle weakness (100% vs 23.5%; p<0.0001) than those without stasis. However, there was no relationship identified with respect to socio-economic status, education, religion, family history and hemoglobin for patients with GB stasis.
Protein intake and GB ejection fraction
In the postpartum period compared to the third trimester of pregnancy there was a significant increase in the calorie intake from 2175 ± 435 to 2537 ± 503 kcal (p<0.001); protein intake from 61.4 ± 14.3 g/day to 66.7 ± 16.1 g/day (p<0.001) and fat intake from 77 ± 23.6 g/day to 99.8 ± 31.7 g/day (p<0.001). The increase in calorie intake was however, predominantly contributed by fat. The mean fat intake was higher than recommended value in all patients during pregnancy and in 80 of the 81 patients in the postpartum period. However, the protein intake was lower than recommended value during pregnancy in 66.7% of the women and in 74% of women in postpartum period. The protein intake was predominantly of poor biological value and was vegetarian in nature.
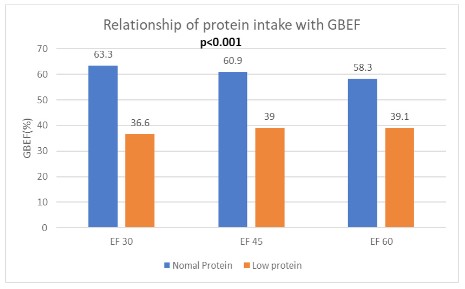
Patients with low protein intake more often had gallstones than those with normal protein intake (13% vs 0%; γ=0.004). Patients with low protein intake also had significantly lower mean peak ejection fraction compared to those with normal protein intake (49.41 ± 15.3% vs 69 ± 13.7%; p<0.001). The ejection fraction was also significantly lower in the low protein group: 30 min (36.6 ± 17.1 vs 63.3 ± 13.43; p<0.001); 45 min (39 ± 19 vs 60.86 ± 15.2; p<0.001) and 60 min (39.1 ± 20.9 vs 58.3 ± 20.16; p<0.001) (Figure 2).
Independent predictors of GBEF
Linear regression analysis identified BMI and total calorie intake as the two independent predictive factors of GBEF. Lower BMI and lower calorie intake were associated with poor ejection fraction. The linear regression equation is as given below:
(1.1 x BMI) + (Total Calories / 1000) + 2.6 = GBEF
The confidence interval for the coefficient of BMI is 0.13-2.1, p=0.027 and confidence interval for the coefficient of calorie intake is 0.003-0.018, p=0.008.
Table 1: Comparison of gall bladder function on USG in post-partum women with healthy.
| Characteristics | Post-partum women (n=81) | Controls (n=22) | p-value |
|---|---|---|---|
| GB ejection fraction (%)* | 54.5 ± 17 | 63.4 ± 8.5 | <0.001 |
| GB fasting volume* | 21.88 ± 10.7 | 16.5 ± 5.2 | 0.002 |
| Residual GB volume at 60 mins* | 11.2 ± 6.4 | 5.8 ± 1.3 | <0.001 |
| GB stasis | 25% | 0% | 0.009 |
controls*- Values expressed in mean ± SD
Table 2: Different factors associated with GB stasis in post-partum women.
| Characteristics | Post-partum women (n=81) | Controls (n=22) | p-value |
|---|---|---|---|
| Characteristics | With GB stasis (n=20) | Without GB stasis (n=61) | p-value |
| BMI* | 21.4 ± 3.7 | 23.8 ± 3.6 | 0.013 |
| Body weight*(kg) | 50.8 ± 11.5 | 57.9 ± 8.5 | 0.004 |
| Height*(cm) | 153 ± 7.7 | 156 ± 6.6 | 0.141 |
| Waist circumference*(cm) | 79 ± 13 | 86 ± 9.7 | 0.014 |
| Waist circumference*(cm) | 79 ± 13 | 86 ± 9.7 | 0.014 |
| Hip circumference*(cm) | 90 ± 9 | 96 ± 7 | 0.003 |
| Total calorie intake*(Kcal) | 2237 ± 289 | 2636 ± 521 | <0.001 |
| Total Fat intake (gm) | 84.9 ± 20 | 104.6 ± 33 | 0.015 |
| Total Fat intake (gm) | 84.9 ± 20 | 104.6 ± 33 | 0.015 |
| Daily protein intake* (gm) | 58 ± 10 | 69.4 ± 16.8 | <0.001 |
| Triceps fold thickness* (mm) | 18 ± 5 | 21 ± 5.3 | 0.035 |
| Biceps fold thickness* (mm) | 7.7 ± 2.5 | 10.3 ± 4.1 | 0.001 |
controls*- Values expressed in mean ± SD
Table 3: Different factors associated with GB stasis in post-partum women.
| Characteristics | WWith gall stones (n=8) | Without gall stones (n=73) | p-value |
|---|---|---|---|
| Multi-parity (%) | 75% | 22% | 0.001 |
| Serum ferritin levels* (μg/L) | 20.3±10.5 | 41.7±38 | 0.001 |
| Total daily calorie*intake (Kcal) | 2266±269 | 2567±515 | 0.19 |
| Daily fat intake*(gm) | 91±17.2 | 100±32.8 | 0.22 |
| Daily protein intake* (gm) | 60.7±9.49 | 67.4±16.6 | 0.115 |
Gallstones and sludge
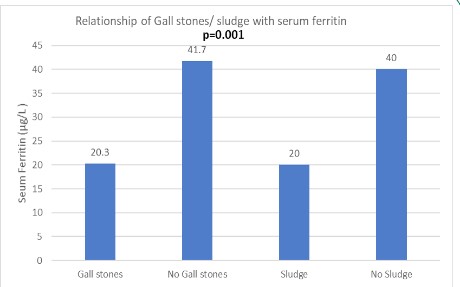
Postpartum women with GS (n=8) more often had associated sludge in contrast to those without GS (n=73) (37.5% vs 1.3%; p=0.002). Patients with GS were more often multiparous compared to those without GS (75% vs 22%; p=0.001). Patients with GS more often had history of biliary colic during pregnancy (37.5% vs 6.8%; p=0.006) and had significantly lower mean serum ferritin levels (20.3 ± 10.5 vs 41.7 ± 38 μg/L; p=0.001) (Table 3, Figure 3). Patients with sludge (n=4) had lower serum ferritin levels than those without sludge (n=77) (20 ± 2 vs 40 ± 37; p<0.001). However, there was no relationship identified with respect to socio-economic status, education, religion, family history, hemoglobin, calorie intake and lipid profile for patients with GS or GB sludge.
Parity
Most of the patients (75%) with gallstones were multiparous. Multiparous women (n=22) were more likely to have GS than primiparas (n=59) (27% vs 3%; p<0.001). The GBEF was similar in multiparous and primiparous women (54.8 ± 17.3 vs 53.49 ± 17.2; p>0.05). The lipid profile was similar in both the groups.
Discussion
We found that postpartum women at 6 weeks after delivery had higher prevalence of gallbladder stasis with higher basal gallbladder volumes, poorer ejection fraction and sluggish response to fatty meal. Post-partum women with GB stasis were found to have lower body weight, lower BMI and lower mean daily protein intake compared to patients without stasis. The prevalence of gallstones among our postpartum patients (10%) was similar to that reported in previous studies from the west (2-12%) [3,12]. The incidence of sludge (5%) observed in our study is similar to that reported from Netherlands [13]. Previous studies have shown though the mean basal volume is about 70% higher in pregnant females as compared to non-pregnant controls, and the volumes return back to normal as early as 2 weeks postpartum [13,14]. However, our patients even at 6 weeks postpartum exhibited larger volumes and stasis which may be due to poor protein intake in their diet, lower BMI or concomitant iron deficiency.
We found that majority of our study population had lower protein intake and higher fat intake than that recommended values for postpartum state [9]. We found statistically significant association between low protein intake in postpartum period with poor peak ejection fraction as well as ejection fraction at 15, 30, 45 and 60 minutes post meals. Low protein intake is also associated with increased risk of GB stasis and gallstones. Protein is an independent stimulant for the release of CCK. Low protein diet thus may be associated with inappropriately low CCK release resulting in cumulative stasis leading to increased gallstone formation. It has been earlier demonstrated that the CCK levels are lower and gastric emptying is slower in rats fed on chronic low protein diet in contrast to rats on fed on high protein diet [15]. Low protein intake possibly results in also decreased smooth muscle responsiveness to cholecystokinin (CCK). Earlier studies have also shown increased risk of cholecystectomy in patients with low intake of vegetable protein along with energy-balanced diet [16].
We found that patients with sludge or GS had significantly lower serum ferritin levels as compared to those without sludge or GS. The prevalence of iron deficiency was alarming high which may be due to poor compliance for oral iron, poor baseline iron status prior to the index pregnancy, co-existent worm infestation, poor availability of iron in Indian diet, multiple pregnancies with poor spacing and vegetarian diet [17]. Various mechanisms have been postulated regarding role of iron deficiency in gallstone pathogenesis [18]. Johnston et al., has shown that 80% of adult male prairie dogs on iron deficient diet for 8 weeks developed cholesterol monohydrate crystals in bile and 30% developed gallstones [5]. They have also shown that patients with gallstones had increased biliary transferrin which is a potent nucleator of cholesterol crystals [5]. Subsequently, Swartz-Basile et al., showed that iron deficiency leads to diminished levels of neural nitric oxide synthase in gallbladder predisposing to gallbladder stasis [6]. In a study by Pamuk G E et al., patients with iron deficiency anemia were more likely to have gall stones and impaired GB motility leading higher residual volume [19]. In our study patients with GS or sludge had lower ferritin levels compared to patients without GS or sludge. In our study iron deficiency was not associated with GB stasis or altered GB ejection fraction. However, due to small sample size it requires validation in larger multicentric trials.
In our study, majority of patients were having normal BMI. In earlier studies, Maringhini et al had found that obesity was an independent risk factor for gallstone disease [3]. The fact that a large proportion of our patients with GS have a normal BMI, suggests that imbalanced diet and malnutrition are the primary contributors to the pathogenesis rather than obesity per say at the time of pregnancy.
The mean parity in gallstone group was higher (2.70 ± 1.25 versus 2.0 ± 1.09) than the non-gallstone group and GS were more common among the multiparous. We found that multiparity predisposes to GS as found in other studies [2,20]. Though the incidence of gallstones was higher among the multiparous compared to primiparas, the incidence among the primiparas was also significantly higher than what is expected. Tsimoyiannis et. al had shown that women who have three or more pregnancies were more likely to develop gallstones as compared to nulliparous women (p=0.01) [21]. Similarly Gilat et al found that the incidence of gallstones in multiparous females is 19% as compared to 8% in nulliparous women [22]. Thus, there was a trend towards increased risk of gallstones with increasing parity and this observation is similar to that found in earlier studies. Multiple poorly spaced out pregnancy among our multiparous women possibly predisposes our patients to multiple nutrient deficiencies. Various local cultural practice during post-partum period like diet with high fat, low protein and low iron put post partum women at a higher risk of gall bladder stasis and gall stone formation.
In our study follow-up of patients was not done to document the long-term outcomes of altered GB motility and GB stasis. Certain groups like patients with gall stones or sludge was small so implication of results on a larger scale requires larger, prospective multicentric studies.
Overall, this study highlights that women in the postpartum period who are malnourished, iron deficient, multiparous and consuming protein deficient diet are at higher risk for gallbladder stasis and to develop gallstones and sludge. It would be thus appropriate to ensure that women during their pregnancy and postpartum period should be instructed to consume a balanced diet rich in proteins and iron.
Declarations
Grant support: Gr None.
Conflicts of Interest: None.
Acknowledgements: We would like to thank Mr. Jitender Kumar and Sunil Kumar, for assisting in preparation of the manuscript.
References
- Tandon RK. Pathogenesis of gallstones in India. Trop Gastroenterol. 1988 ; 9: 83-93.
- Attili AF, Capocaccia R, Carulli N, Festi D, Roda E, et al. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatol Baltim Md. 1997; 26: 809-818.
- Maringhini A, Ciambra M, Baccelliere P, Raimondo M, Orlando A, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med. 1993; 119: 116-120.
- Lynn J, Williams L, O’Brien J, Wittenberg J, Egdahl RH. Effects of estrogen upon bile: implications with respect to gallstone formation. Ann Surg. 1973; 178: 514-524.
- Johnston SM, Murray KP, Martin SA, Fox-Talbot K, Lipsett PA, et al. Iron deficiency enhances cholesterol gallstone formation. Surgery. 1997; 122: 354-361; discussion 361-362.
- Swartz-Basile DA, Goldblatt MI, Blaser C, Decker PA, Ahrendt SA, et al. Iron deficiency diminishes gallbladder neuronal nitric oxide synthase. J Surg Res. 2000; 90: 26-31.
- Misciagna G, Centonze S, Leoci C, Guerra V, Cisternino AM, et al. Diet, physical activity, and gallstones--a population-based, case-control study in southern Italy. Am J Clin Nutr. 1999; 69: 120-126.
- Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999; 9: 178-187.
- Singh RB, Rastogi SS, Rao PV, Das S, Madhu SV, et al. Diet and lifestyle guidelines and desirable levels of risk factors for the prevention of diabetes and its vascular complications in Indians: a scientific statement of The International College of Nutrition. Indian Consensus Group for the Prevention of Diabetes. J Cardiovasc Risk. 1997; 4: 201-208.
- Dodds W, Groh W, Darweesh R, Lawson T, Kishk S, et al. Sonographic measurement of gallbladder volume. Am J Roentgenol 1985; 145: 1009–1011.
- Toouli J. Biliary Dyskinesia. Curr Treat Options Gastroenterol. 2002; 5: 285-291.
- Ko CW, Beresford SAA, Schulte SJ, Matsumoto AM, Lee SP. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatol Baltim Md. 2005; 41: 359-365.
- Van Bodegraven AA, Böhmer CJ, Manoliu RA, Paalman E, Van der Klis AH, Roex AJ et al. Gallbladder contents and fasting gallbladder volumes during and after pregnancy. Scand J Gastroenterol. 1998; 33: 993-997.
- Hahm JS, Park JY, Song SC, Cho YJ, Moon KH, et al. Gallbladder Motility Change in Late Pregnancy and after Delivery. Korean J Intern Med. 1997; 12: 16-20.
- Shi G, Leray V, Scarpignato C, Bentouimou N, Bruley des Varannes S, et al. Specific adaptation of gastric emptying to diets with differing protein content in the rat: is endogenous cholecystokinin implicated? Gut. 1997; 41: 612-618.
- Tsai C-J, Leitzmann MF, Willett WC, Giovannucci EL. Dietary protein and the risk of cholecystectomy in a cohort of US women: the Nurses’ Health Study. Am J Epidemiol. 2004; 160: 11-18.
- Narasinga Rao BS, Vijayasarathy C, Prabhavathi T. Iron absorption from habitual diets of Indians studied by the extrinsic tag technique. Indian J Med Res. 1983; 77: 648-657.
- Prasad PC, Gupta S, Kaushik N. To study serum iron levels in patients of gall bladder stone disease and to compare with healthy individuals. Indian J Surg. 2015; 77: 19-22.
- Pamuk GE, Umit H, Harmandar F, Yeşil N. Patients with iron deficiency anemia have an increased prevalence of gallstones. Ann Hematol. 2009; 88: 17-20.
- Johnston DE, Kaplan MM. Pathogenesis and treatment of gall-stones. N Engl J Med. 1993; 328: 412-421.
- Tsimoyiannis EC, Antoniou NC, Tsaboulas C, Papanikolaou N. Cholelithiasis during pregnancy and lactation. Prospective study. Eur J Surg Acta Chir. 1994; 160: 627-631.
- Gilat T, Konikoff F. Pregnancy and the biliary tract. Can J Gastroenterol. 2000; 14: 55D-59D.