Journal of Gastroenterology Research and Practice
Research Article - Open Access, Volume 3
Impact of low- and standard-abdominal pressure on post- operative pain during laparoscopic cholecystectomy: A systematic review and meta-analysis of randomized trials
Jing Li1,2; Xiaojun Yang1,2; Naijun Chai1,2; Kongyuan Wei3; Zebin Jiang1,2*
1The 2nd Department of General Surgery, Gansu Provincial Hospital, Lanzhou, Gansu, China
2Gansu Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology, Gansu Provincial Hospital,
Lanzhou, Gansu, China.
3Department of General, Visceral and Transplantation Surgery, University of Heidelberg, Heidelberg, Germany
*Corresponding Author : Zebin Jiang
The 2nd Department of General Surgery, Gansu Provincial Hospital, Lanzhou, Gansu, China.
Tel: +14693638905;
Email: bbobb_001@163.com
Received : Jan 10, 2023
Accepted : Mar 02, 2023
Published : Mar 09, 2023
Archived : www.jjgastro.com
Copyright : © Jiang Z (2023).
Abstract
Background: This study aimed to comprehensively evaluate the impact of low- and standard-abdominal pressure on intraoperative, postoperative, and survival outcomes of Laparoscopic Cholecystectomy (LC).
Methods: A literature search of the databases, PubMed, Web of Science, Embase, and Cochrane Library was performed until April 30, 2021. Studies comparing low abdominal pressure and standard abdominal pressure for LC were included. Two reviewers independently screened the studies, extracted the data of interest, and assessed the risk of bias. Meta-analysis was performed using RevMan 5.3.
Results: Thirty-seven RCTs, including 2,104 patients, met the eligibility criteria. Low-abdominal pressure showed lower shoulder pain (RR: 0.55, 95% CI: 0.46, 0.67; p<0.001), lower analgesic use (RR: 0.54, 95% CI: 0.30, 0.99; p=0.039), lower pain score 0-6 h after operation (SMD: -0.28, 95% CI: -0.57, 0.00; p=0.04), lower pain score 7-12 h after operation (SMD: -0.81, 95% CI: -1.35, -0.28; p=0.003), lower pain score 13-24 h after operation (SMD: -0.66, 95% CI: -1.06, -0.26; p=0.001), longer operative time (WMD:1.52, 95% CI: 0.26, 2.78, p =0.02), and length of hospital stay (WMD: -0.31, 95% CI: -0.54, -0.07; p=0.01). Conversion to open surgery (RR, 0.86; 95% CI: 0.28, 2.64; p=0.79) was not significantly different between the two groups.
Conclusion: Low abdominal pressure showed impressive improvement of shoulder pain and reduction of analgesic use. The pain score after surgery and length of hospital stay was also improved compared with standard abdominal pressure. The conclusions should be interpreted with caution because of the moderate to high degree of heterogeneity.
Keywords: Laparoscopic cholecystectomy; Abdominal pressure; Meta-analysis.
Abbreviations: LAP: Low Abdominal Pressure; LC: Laparoscopic Cholescystectomy; SAP: Standard Abdominal Pressure.
Citation: Li J, Yang X, Chai N, Wei K, Jiang Z. Impact of low- and standard-abdominal pressure on post-operative pain during laparoscopic cholecystectomy: A systematic review and meta-analysis of randomized trials. J Gastroenterol Res Pract. 2023; 3(1): 1129.
Introduction
Laparoscopic Cholecystectomy (LC) has been shown to be more beneficial than traditional cholecystectomy for patients with cholecystitis [1]. As a minimally invasive technique, LC improves the quality of postoperative living conditions and decreases the overall complications. LC is strongly recommended by the clinical guidelines worldwide [2]. However, some risk factors may affect the safety and efficacy of LC, especially abdominal pressure, which is considered to be one of the most important factors influencing postoperative complications [3].
For laparoscopic surgery, gas abdominal pressure caused by the input air is inevitable. Hence, postoperative complications, such as shoulder pain, may significantly affect the patient’s quality of life [4,5]. Moreover, postoperative pain is the most significant complication according to patients with LC [5]. To date, the relationship between abdominal pressure and pain is not clear. The severity of the postoperative pain is considered to be relative to the abdominal pressure [5].
In this review, abdominal pressure of LC was divided into two: Low Abdominal Pressure (LAP) and Standard Abdominal Pressure (SAP). Some minor corrections to the subgroups were made due to the information reported in the included studies [5]. According to recent LC guidelines, the pressure of the LAP and SAP groups was defined as 6–10 mmHg and 12–15 mmHg, respectively [5]. The latest meta-analyses [3,4] suggest that there is a lack of robust evidence on which type of abdominal pressure is beneficial for patients during LC.
Our study aimed to systematically evaluate and compare the impact of the two types of abdominal pressure on intraoperative, postoperative, and survival outcomes in patients who underwent laparoscopic cholecystectomy.
Materials and methods
Study registration
This study was registered on PROSPERO, and the registration number is CRD42018093851.
Study selection
We searched the databases, PubMed, Web of Science, Embase, and Cochrane Library, from their inception to May 31, 2019. The search was updated on April 30, 2021. Studies comparing low and standard abdominal pressure for LC were included. To search comprehensively and systematically for eligible studies, we also performed a manual search of the references found in the published reviews and articles.
Inclusion and exclusion criteria
The inclusion criteria for this meta-analysis were as follows: (1) randomized controlled trials (LAP versus SAP), (2) studies with a total sample size of more than 20, (3) studies where intraoperative and/or postoperative outcomes and/or survival outcomes were reported, and (4) LC patients. The exclusion criteria were as follows: (1) review articles, (2) correspondences or editorials, (3) conference abstracts without detailed data, (4) studies that included patients who underwent open cholecystectomy, (5) animal studies, and (6) single-arm studies.
Data extraction
Two authors (ZGR and LYF) independently reviewed all the identified studies. They resolved discrepancies by discussions. A third reviewer (ZZY) was consulted as necessary. We extracted the following items from each study: first author’s name, year of publication, country, publication type, and study type. There were no limitations in terms of language, race, and gender.
Outcomes of interest
The primary outcomes included shoulder pain, analgesic use, pain score 0-6 h after operation, pain score 7-12 h after operation, and pain score 13-24 h postoperatively. Secondary outcomes included conversion to open surgery, operative time, and length of hospital stay.
Statistical analysis
For dichotomous variables, Risk Ratios (RRs) were calculated with 95% Confidence Intervals (CIs). Continuous parameters, such as operative time and length of hospital stay, were analyzed using the Weighted Mean Difference (WMD). For outcomes such as pain scores, where different scales may have been used, the Standardized Mean Difference (SMD) was applied. We estimated the degree of heterogeneity among studies using the Cochrane Q statistic (p<0.10 was considered representative of statistically significant heterogeneity) and the I2 statistic (I2>50% was considered to represent significant heterogeneity). Data were pooled using the random-effects model if significant heterogeneity among the studies was present. Otherwise, we used a fixed-effects model. We drew funnel plots to determine the possibility of publication bias when more than 10 studies were included. All data were analyzed using RevMan 5.3.
Results
Literature search
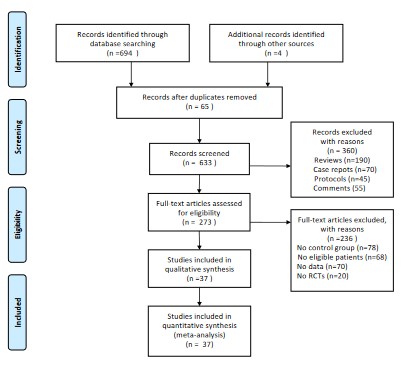
The search yielded 698 records. We excluded 65 studies because of duplication. We assessed the titles and abstracts of 633 studies and excluded 360 studies that did not meet the inclusion criteria. We assessed the full texts of 273 studies and finally included 37 studies for the present systematic review and meta-analysis. The flowchart is shown in Figure 1.
Characteristics of eligible studies
Thirty-seven RCTs and 2104 patients were included. All the included studies compared low- (1022 patients) to standard abdominal pressure (1082 patients) during LC. We redefined the LAP as 6-10 mmHg and SAP as 12-15 mmHg. Two RCTs presented data of patients from America, 11 RCTs provided data of patients from Europe, and 18 RCTs described patients from Asia. Of these, 17 lower abdominal pressure studies (≤8 mmHg) accounted for 50% of all the included studies. The analgesic procedures for all included studies were similar, in that general anesthesia was performed with fentanyl, remifentanil, or morphine. The details are presented in Table 1.
Table 1: Characteristics of eligible studies.
| Study ID | Country | Study design | No. of patients | Sample size | Age, mean (range or SD), y | Female (%) | Pressure (mHg) | JADAD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAP | SAP | LAP | SAP | LAP | SAP | LAP | SAP | |||||
| Pier 1994 [6] | Germany | RCT | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5 |
| Unbehaum 1995 [7] | UK | RCT | NA | NA | NA | NA | NA | NA | NA | NA | 15 | 4 |
| Wallace 1997 [8] | UK | RCT | 40 | 20 | 20 | 59 (52–64) | 56 (50–64) | 14 (70) | 16 (80) | 7.5 | 15 | |
Primary outcomes
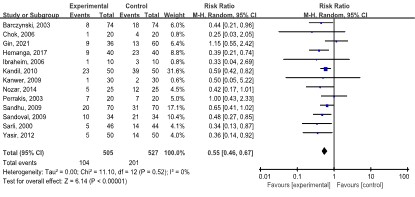
Shoulder pain: Thirteen studies [10-12,19,20,23,24,27, 29,32,34,39-42] including 1036 patients reported shoulder pain outcomes. Of the 505 patients in the LAP group, 104 patients suffered shoulder pain. In the SAP group of 527 patients, 201 reported shoulder pain. The meta-analysis showed that patients in the LAP group had lower shoulder pain than patients in the SAP group (RR: 0.55, 95% CI: 0.46, 0.67; p<0.00001). No statistical heterogeneity (I2=0.0%, p=0.520) was found (Figure 2).
Analgesic use
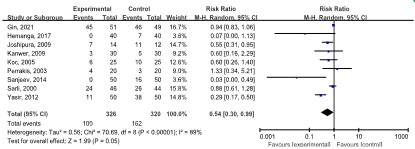
Analgesic use was reported in nine studies [10,12,17, 22,23,32,36,39] including 646 patients. Of the 326 patients in the LAP group, 100 used analgesics. In the SAP group of 320 patients, 162 used analgesics. The meta-analysis revealed that those in the LAP group had a lower analgesic use than patients in the SAP group (RR: 0.54, 95% CI: 0.30, 0.99; p=0.05). A moderate degree of heterogeneity (I2=89.0%, p<0.00001) was noted (Figure 3).
Pain score 0-6 h after operation
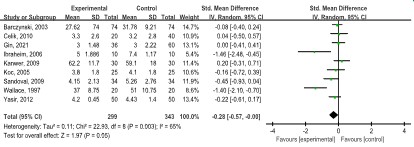
Nine studies [8,11,17,20,23,27,28,32] showed lower pain scores 0-6 h after operation in the LAP group (n=646, SMD: -0.28, 95% CI: -0.57, 0.00; p=0.05), with a moderate degree of heterogeneity (I2=65.0%, p=0.003) (Figure 4).
Pain score 7-12 h after operation
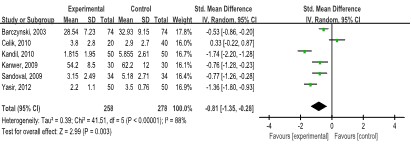
Pain score 7-12 h after operation was presented in six studies [11,23,27-29,32] (536 patients). Patients in the LAP group showed a lower pain score than those in the SAP group (SMD: -0.81, 95% CI: -1.35, -0.28; p=0.003), with a high degree of heterogeneity (I2=88.0%, p<0.00001) (Figure 5).
Pain score 13-24 h after operation
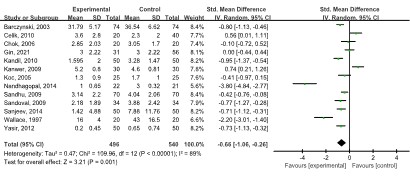
Thirteen studies [8,11,17,19,23,24,27-29,32,35,36] with 1,049 patients reported a lower pain score 13-24 h after operation in the LAP group than in the SAP group (SMD: -0.66, 95% CI: -1.06, -0.26; p=0.001), with a high degree of heterogeneity (I2=89.0%, p<0.00001) (Figure 6).
Secondary outcomes
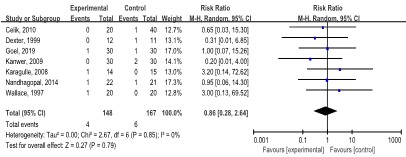
Conversion to open surgery: Seven studies [8,9,21,23,28,35] with 315 patients revealed no statistical significance in the conversion to open surgery between the LAP and SAP groups (RR: 0.86, 95% CI: 0.28, 2.64; p=0.79), without statistical heterogeneity (I2=0.0%, p=0.85) (Figure 7).
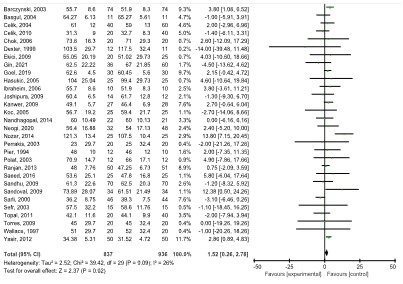
Operative time: Thirty studies [6,8-20,22-28,30,32-35,37] with 1,777 patients reported the mean operative time. The LAP group had a longer operative time than the SAP group (WMD:1.52, 95% CI: 0.26, 2.78, p=0.02), with a low degree of heterogeneity (I2=26.0%, p=0.09) (Figure 8).
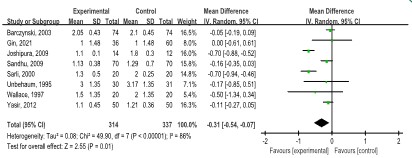
Length of hospital stay: The length of hospital stay was reported in eight studies [7,8,10,11,22,24,32] (655 patients). The LAP group showed a shorter length of hospital stay than the SAP group (WMD: -0.31, 95% CI: -0.54, -0.07; p=0.01), with a high degree of heterogeneity (I2=86.0%, p<0.00001) (Figure 9).
Publication bias
Three outcomes (shoulder pain, pain score 13-24 h after operation, and operative time) included more than 10 studies. Funnel plots suggested that the possibility of publication bias was small.
Discussion
This meta-analysis indicated that the LAP group was more beneficial for lower shoulder pain, analgesic use, pain score 0-6 h after operation, pain score 7-12 h after operation, pain score 13-24 h after operation, and length of hospital stay. Although, the operative time of the LAP group was longer. Other outcomes, such as conversion to open surgery, were not significantly different between the two types of abdominal pressure for LC. Most of the results attained a moderate to high degree of heterogeneity due to some inevitable bias; hence, the evidence may not be robust enough for clinical practice.
Interestingly, the LAP group had lower shoulder pain than the SAP group in most of the included studies, which suggests that there was no heterogeneity. Shoulder pain results from the pressure elicited by the injected gas in the abdomen. Although LC is widely popular worldwide, the possible postoperative complications remain confusing to many clinical workers [43]. Shoulder pain is one of the inevitable postoperative complications that influence the patient’s quality of life in a large part.
Hence, it is necessary to choose a favorable abdominal pressure to reduce the incidence of shoulder pain. Given these results, LAP may minimize shoulder pain.
The LAP group also indicated a lower pain score 0-6 h after the operation, a pain score of 7-12 h, and a pain score of 13-24 h postoperatively. Pain score was evaluated using the visual analog scale (VSA) score, where 0 represents no pain and 10 represents maximum pain. VSA assesses the severity of post-operative complications approximately [44]. A high degree of heterogeneity may be generated due to different ways of evaluating pain in the included studies. The outcomes of pain score 0-6 h, pain score 7-12 h after operation, and pain score 13-24 h after operation were associated with a high degree of heterogeneity. Hence, the results should be interpreted with caution.
Analgesic use was also evaluated in this meta-analysis. The LAP group used less analgesic than the SAP group. This was associated with a moderate degree of heterogeneity. Analgesics are commonly used to relieve the discomfort caused by pain. Although LC is minimally invasive, postoperative pain does occur to some extent. The lower incidence of analgesic use may improve the quality of life for the patients with LC.
Operative time and length of hospital stay were compared between the two groups. Patients in the LAP group had a shorter operative time and length of hospital stay. The length of hospital stay was presented with a high degree of heterogeneity as most studies that did not define the length of hospital accurately and concisely. Moreover, the two groups had no significant difference in the conversion to open surgery, which showed no benefit in either group.
Surprisingly, shoulder pain, pain score 0-6 h after operation, pain score 7-12 h after operation, pain score 13-24 h after operation, and analgesic use all indicated that LAP was beneficial for patients who underwent LC. Due to the lower abdominal pressure, the incidence of pain decreased and quality of life improved. As such, LAP for LC should be recommended for clinical practice. The operative time and length of hospital stay were also shortened in the LAP group, suggesting that LAP may generate fewer complications and enhance the recovery of patients after LC.
Recently published meta-analysis analyses [3,4] reported that LAP is more beneficial than SAP but the outcomes were not analyzed systematically. We included the newly published RCTs from 2014 to 2018 and analyzed the data comprehensively in this meta-analysis. The results indicated that LAP is not inferior to SAP. Furthermore, LAP may lead to better safety and efficacy. Well-conducted and updated meta-analyses of RCTs are accepted as the best-quality evidence for informing clinical practice and health policy [45,46]. However, there are still some unsolved questions when comparing LC using LAP versus SAP. The incidence of malignant heart events was not shown in this meta-analysis because there was not enough data to describe the cardiovascular problems in patients who underwent LC. Therefore, more LC studies focusing on cardiovascular problems are needed. Cardiovascular events are common complications after inputting gas into the abdomen, and the extra pressure may influence the working of the heart [43]. Besides, cardiovascular problems significantly impact the quality of life of patients after laparoscopic surgery [47]. More studies should focus on these questions in the future to improve the quality of life for patients after LC.
The findings of this study indicated that LAP has obvious advantages compared with SAP, although most outcomes were associated with a high degree of heterogeneity. The heterogeneity may come from different pain evaluation methods, different populations, different operations performed by different surgeons, and so on. In sum, LAP for LC improves intraoperative and postoperative complications, without affecting the surgeons’ visual and physical access. In other words, lower pres- sure may provide a wide and clear view during the operation.
Considering the recent clinical guidelines [48,49] on laparoscopic surgery, a few have recommended LAP for patients with gall bladder diseases. Previous studies [6-8] have suggested that LAP may not provide an obvious and high-quality view of the operative field because low abdominal pressure may not provide enough space for the surgeons. In contrast, according to our results, the use of LAP for the operation did not hinder access, regardless of the heterogeneity, and that LAP may not offer a larger operative space. To the best of our knowledge, LAP provides adequate space for the surgeons, as well as better safety and efficacy. However, our results need to be validated by high-quality prospective studies.
There are some limitations in this meta-analysis. (1) Some heterogeneity may influence the results to some extent. We did not perform the certainty of evidence assessment using the GRADE system because of the lack of skill and experience with the GRADE method [50]. (2) Some of the included studies had inevitable biases, which may affect the quality of the evidence. (3) Due to sufficient data, some outcomes could not be metaanalyzed to offer more comprehensive evidence for LC.
Some merits were also shown in this meta-analysis. (1) All the included studies were randomized controlled trials comparing LAP and SAP. (2) Almost all the necessary outcomes, including intraoperative and postoperative complications, were analyzed in this meta-analysis. (3) This is the first meta-analysis comparing LAP and SAP with systematic data and comprehensive RCTs published until 2019. In addition, our search was guided by an experienced information expert who improved the search quality [51].
Conclusions
LAP showed impressive improvement of shoulder pain and reduction of analgesic use. LAP was also associated with improved pain score after surgery and length of hospital stay compared with that of standard abdominal pressure. The conclu- sions should be interpreted with caution, and in combination with clinical practice experience, because most of the results had a high degree of heterogeneity.Declarations
Conflict of interest: The authors have no conflicts of interest to declare.
Funding: Health industry research project of Gansu Province-GSWSKY2021-060, Research Fund project of Gansu Provincial People's Hospital-21GSSYC-3, Science and Technology Project of Gansu Province-20JR10RA402.
Authors’ contributions: JL, XJY, NJC and ZBJ designed the study. JL and KYW drafted the protocol, with assistance from XJY. JL will perform the literature search. JL and XJY will carry out the data collection and NJC will assist with data collection as the third reviewer. JL, XJY and NJC will perform quality assessment. JL will complete the data analysis with statistical assistance from XJY and NJC. JL, XJY and NJC will draft the final manuscript, which will be reviewed by all co-authors. ZBJ will be the guarantor of the review. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of this work.
Acknowledgments: None.
References
- Schirmer BD, Edge SB, Dix J, Hyser MJ, Hanks JB, et al. Laparoscopic cholecystectomy. Treatment of choice for symptomatic cholelithiasis. Ann Surg. 1991; 213: 665-676.
- Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006; 4: CD006231.
- Gurusamy KS, Vaughan J, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014; 3: CD006930.
- Hua J, Gong J, Yao L, Zhou B, Song Z. Low-pressure versus standard-pressure pneumoperitoneum for laparoscopic cholecystectomy: a systematic review and meta-analysis. Am J Surg. 2014; 208: 143-150.
- Sarli L, Costi R, Sansebastiano G, Trivelli M, Roncoroni L. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg. 2000; 87: 1161-1165.
- Pier A, Benedic M, Mann B, Buck V. Postlaparoscopic pain syndrome. Results of a prospective, randomized study. Chirurg. 1994; 65: 200-208.
- Unbehaum N, Feussner H, Siewert JR. Low-pressure insufflation technique in the laparoscopic cholecystectomy. Minim Invas Chir. 1995; 4: 10-15.
- Wallace DH, Serpell MG, Baxter JN, O’Dwyer PJ. Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg 1997; 84: 455-458.
- Dexter SP, Vucevic M, Gibson J, McMahon MJ. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999; 13(4):376-381.
- Sarli L, Costi R, Sansebastiano G, Trivelli M, Roncoroni L. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg 2000;87(9):1161-1165.
- Barczyński M, Herman RM. A prospective randomized trial on comparison of low-pressure (LP) and standard-pressure (SP) pneumoperitoneum for laparoscopic cholecystectomy. Surg Endosc. 2003; 17: 533-538.
- Perrakis E, Vezakis A, Velimezis G, Savanis G, Deverakis S, et al. Randomized comparison between different insufflation pressures for laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2003;13: 245-249.
- Polat C, Yilmaz S, Serteser M, Koken T, Kahraman A, et al. The effect of different intraabdominal pressures on lipid peroxidation and protein oxidation status during laparoscopic cholecystectomy. Surg Endosc. 2003; 17: 1719-1722.
- Sefr R, Puszkailer K, Jagos F. Randomized trial of different intraabdominal pressures and acid–base balance alterations during laparoscopic cholecystectomy. Surg Endosc. 2003; 17: 947-950.
- Basgul E, Bahadir B, Celiker V, Karagoz AH, Hamaloglu E, et al. Effects of low and high intraabdominal pressure on immune response in laparoscopic cholecystectomy. Saudi Med J. 2004; 25: 1888-1891.
- Celik V, Salihoglu Z, Demiroluk S, Unal E, Yavuz N, et al. Effect of intra-abdominal pressure level on gastric intramucosal pH during pneumoperitoneum. Surg Laparosc Endosc Percutan Tech. 2004; 14: 247-249.
- Koc M, Ertan T, Tez M, Kocpinar MA, Kilic M, et al. Randomized, prospective comparison of postoperative pain in low- versus high-pressure pneumoperitoneum. ANZ J Surg. 2005; 75: 693-696.
- Hasukić S. Postoperative changes in liver function tests: randomized comparison of low- and high-pressure laparoscopic cholecystectomy. Surg Endosc. 2005; 19: 1451-1455.
- Chok KS, Yuen WK, Lau H, Fan ST. Prospective randomized trial on low-pressure versus standard-pressure pneumoperitoneum in outpatient laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2006; 16: 383-386.
- Ibraheim OA, Samarkandi AH, Alshehry H, Faden A, Farouk EO. Lactate and acid base changes during laparoscopic cholecystectomy. Middle East J Anaesthesiol. 2006; 18: 757-768.
- Karagulle E, Turk E, Dogan R, Ekici Z, Dogan R, Moray G. The effects of different abdominal pressures on pulmonary function test results in laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2008; 18: 329-333.
- Joshipura VP, Haribhakti SP, Patel NR, Naik RP, Soni HN, et al. A prospective randomized, controlled study comparing low pressure versus high pressure pneumoperitoneum during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2009; 19: 234-240.
- Kanwer DB, Kaman L, Nedounsejiane M, Medhi B, Verma GR, et al. Comparative study of low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy--a randomised controlled trial. Trop Gastroenterol. 2009; 30: 171-174.
- Sandhu T, Yamada S, Ariyakachon V, Chakrabandhu T, Chongruksut W, et al. Low-pressure pneumoperitoneum versus standard pneumoperitoneum in laparoscopic cholecystectomy, a prospective randomized clinical trial. Surg Endosc. 2009; 23: 1044-1047.
- Torres K, Torres A, Staśkiewicz GJ, Chrościcki A, Loś T, et al. A comparative study of angiogenic and cytokine responses after laparoscopic cholecystectomy performed with standard- and low-pressure pneumoperitoneum. Surg Endosc. 2009; 23: 2117-2123.
- Ekici Y, Bozbas H, Karakayali F, Salman E, Moray G, et al. Effect of different intra-abdominal pressure levels on QT dispersion in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2009; 23: 2543-2549.
- Sandoval-Jiménez CH, Méndez-Sashida GJ, Cruz-Márquez-Rico LM, Cárdenas-Victorica R, Guzmán-Esquivel H, et al. Postoperative pain in patients undergoing elective laparoscopic cholecystectomy with low versus standard-pressure pneumoperitoneum. A randomized clinical trial. Rev Gastroenterol Mex. 2009; 74: 314-320.
- Celik AS, Frat N, Celebi F, Deniz G, Rafet K, et al. Laparoscopic cholecystectomy and postoperative pain: is it affected by intra-abdominal pressure? Surg Laparosc Endosc Percutan Tech. 2010; 20: 220-222.
- Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A. 2010; 20: 677-682.
- Topal A, Celik JB, Tekin A, Yüceaktaş A, Otelcioğlu S. The effects of 3 different intra-abdominal pressures on the thromboelastographic profile during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2011; 21: 434-438.
- Eryılmaz HB, Memiş D, Sezer A, Inal MT. The effects of different insufflation pressures on liver functions assessed with LiMON on patients undergoing laparoscopic cholecystectomy. Scientific World Journal. 2012; 2012: 172575.
- Yasir M, Mehta KS, Banday VH, Aiman A, Masood I, et al. Evaluation of post operative shoulder tip pain in low pressure versus standard pressure pneumoperitoneum during laparoscopic cholecystectomy. Surgeon. 2012; 10: 71-74.
- Gupta R, Kaman L, Dahiya D, Gupta N, Singh R. Effects of varying intraperitoneal pressure on liver function tests during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2013; 23: 339-342.
- Nasajiyan N, Javaherfourosh F, Ghomeishi A, Akhondzadeh R, Pazyar F, et al. Comparison of low and standard pressure gas injection at abdominal cavity on postoperative nausea and vomiting in laparoscopic cholecystectomy. Pak J Med Sci. 2014; 30: 1083-1087.
- Vijayaraghavan N, Sistla SC, Kundra P, et al. Comparison of standard-pressure and low-pressure pneumoperitoneum in laparoscopic cholecystectomy:A double blinded randomized controlled study. Surg Laparosc Endosc Percutan Tech. 2014; 24: 127-133.
- Singla S, Mittal G, Raghav, Mittal RK. Pain management after laparoscopic cholecystectomy-A randomized prospective trial of low pressure and standard pressure pneumoperitoneum. J Clin Diagn Res. 2014; 8: 92-94.
- Shoar S, Naderan M, Ebrahimpour H, Soroush A, Nasiri S, et al. A prospective double-blinded randomized controlled trial comparing systemic stress response in Laparoascopic cholecystectomy between low-pressure and standard-pressure pneumoperitoneum. Int J Surg. 2016; 28: 28-33.
- Sharma A, Dahiya D, Kaman L, Saini V, Behera A. Effect of various pneumoperitoneum pressures on femoral vein hemodynamics during laparoscopic cholecystectomy. Update Surg. 2016; 68: 163-169.
- Bhattacharjee HK, Jalaludeen A, Bansal V, Kumar S, Krishna A, et al. Impact of standard-pressure and low-pressure pneumoperitoneum on shoulder pain following laparoscopic cholecystectomy: a randomised controlled trial. Surg Endosc. 2017; 31: 1287-1295.
- Gin E, Lowen D, Tacey M, Hodgson R. Reduced laparoscopic intra-abdominal pressure during laparoscopic cholecystectomy and its effect on post-operative pain: a double-blinded randomised control trial. J Gastrointest Surg. 2021.
- Goel A, Gupta S, Bhagat TS, Garg P. Comparative analysis of hemodynamic changes and shoulder tip pain under standard pressure versus low-pressure pneumoperitoneum in laparoscopic cholecystectomy. Euroasian J Hepatogastroenterol. 2019; 9: 5-8.
- Neogi P, Kumar P, Kumar S. Low-pressure pneumoperitoneum in laparoscopic cholecystectomy: A randomized controlled trial. Surg Laparosc Endosc Percutan Tech. 2020; 30: 30-34.
- Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive-pressure capnoperitoneum for laparoscopic cholecystectomy: randomized controlled trial. Ann Surg. 2004; 239: 388-394.
- Lee W, Hahn K, Hur J, Kim Y. Effect of topical lidocaine patch on postoperative pain management in laparoscopic appendectomy: A randomized, double-blind, prospective study. J Laparoendosc Adv Surg Tech A. 2018; 28: 1061-1067.
- Ge L, Tian JH, Li YN, Pan JX, Li G, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol. 2018; 93: 45-55.
- Xiu-xia L, Ya Z, Yao-long C, Ke-hu Y, Zong-jiu Z. The reporting characteristics and methodological quality of Cochrane reviews about health policy research. Health Policy. 2015; 119: 503-510.
- Fry DE, Pine M, Nedza S, Locke D, Reband A, et al. Hospital outcomes in inpatient laparoscopic cholecystectomy in medicare patients. Ann Surg 2017; 265: 178-184.
- Schiphorst AH, Verweij NM, Pronk A, Borel Rinkes IH, Hamaker ME. Non-surgical complications after laparoscopic and open surgery for colorectal cancer—A systematic review of randomised controlled trials. Eur J Surg Oncol. 2015; 41: 1118-1127.
- Wakabayashi G, Iwashita Y, Hibi T, Strasberg SM, Asbun HJ, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25: 73-86.
- Han HS, Yoon YS, Agarwal AK, Belli G, Itano O, et al. Laparoscopic surgery for gallbladder cancer: an expert consensus statement. Dig Surg. 2019; 36: 1-6.
- Norris SL, Meerpohl JJ, Akl EA, Schünemann HJ, Gartlehner G, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016; 79: 150-158. e1.