Japanese Journal of Gastroenterology Research
Case Series - Open Access, Volume 2
Metastatic renal cell carcinoma presenting as gastric ulceration: Case series & literature review
Keela R Scott1*; Caitlyn J Smith1; Morgan Seibert2; Nanda D Thimmappa2; Deepthi S Rao1
1Department of Pathology and Anatomical Sciences, University of Missouri School of Medicine, Columbia, Missouri 65212, USA.
2Department of Radiology, University of Missouri School of Medicine, Columbia, Missouri 65212, USA.
*Corresponding Author : Keela R Scott
Department of Pathology and Anatomical Sciences,
University of Missouri School of Medicine, Columbia,
Missouri 65212, USA.
Email: krshr3@health.missouri.edu
Received : Dec 02, 2022
Accepted : Dec 26, 2022
Published : Dec 30, 2022
Archived : www.jjgastro.com
Copyright : © Scott KR (2022).
Abstract
Renal Cell Carcinoma (RCC) accounts for approximately 3% of all adult malignancies. RCC has a propensity to metastasize along the hematogenous route. About 25% of patients with RCC have distant metastases at presentation. True gastrointestinal metastases, specifically to the gastric wall, have been rarely observed. We present four cases of metastatic renal cell carcinoma to the stomach with ulceration was a characteristic feature.
Citation: Scott KR, Smith CJ, Seibert M, Thimmappa ND, Rao DS. Metastatic renal cell carcinoma presenting as gastric ulceration: Case series & literature review. Japanese J Gastroenterol Res. 2022; 2(2): 1126.
Introduction
In 2019, it was estimated that 73,820 people in the United States will be diagnosed with malignancies of the kidney and renal pelvis. Additionally, an estimated 85% of renal tumors are Renal Cell Carcinoma (RCC), and approximately 70% are of the clear cell histology [2]. Approximately one-third of patients diagnosed with RCC present with metastatic disease to the lung (45%), bone (30%), and lymph nodes (22%); however, metastasis to the stomach is less common [1]. For therapy selection, factors such as tumor histology and stage are important. Patients with RCC confined to the primary site at the time of diagnosis have a higher 5-year survival rate (92.5%) compared to those with distant metastases at the time of diagnosis (12%) [2].
Case description
Our investigation identified four renal cell carcinoma (RCC) patients with gastric metastases.
Case #1 is a 65-year-old Caucasian female that initially presented with a right neck mass. Imaging studies, CT scan of the neck and chest, revealed an 8.2 cm right renal mass. She underwent right nephrectomy which revealed clear cell renal cell carcinoma, histologic grade 3 with no capsular invasion and margins free of malignancy. There was no adjuvant treatment at that time. Interestingly, the right neck mass was never biopsied and completely resolved following her right nephrectomy. She developed left rib pain one year later; however, workup was initially negative. Over the course of 4 months the pain became progressive and she was found to have a left rib fracture. Workup at this time found metastatic lung nodules, a pre-tracheal mass, and a left paravertebral retroperitoneal mass. A bone scan revealed abnormal uptake in the skull, T4, T5, L2, and the left upper lateral ribs and sternum. She also had a large lytic L2 lesion. The patient was given 30 Gy radiation in 10 fractions over 14 days to these bone lesions. Two years from her initial diagnosis she presented with dysphagia. The patient subsequently underwent EGD with biopsy of a 1.5-2 cm meaty sessile polypoid lesion in the mid-gastric body. Immunohistochemical stains of the gastric polyp biopsy were positive for vimentin and RCC consistent with metastatic RCC.
Case #2 is a 45-year-old Caucasian male that initially presented with anemia, fatigue and a 60-pound weight loss over an 8-month period. Imaging studies, CT scan of the chest, abdomen, and pelvis, revealed a 13 cm right renal lesion as well as a 1 cm right lower lobe lung lesion. He underwent a right radical nephrectomy which revealed clear cell renal cell carcinoma, histologic grade 4 with no capsular invasion and margins negative for malignancy with the superior surgical margin <1 mm from the tumor. Overall pathologic stage T3a due to extension into perinephric tissue. He also underwent a wedge resection of the right lower lobe lung lesion which revealed metastatic carcinoma. The patient was then started on high-dose IL-2 of which he received two cycles after discovery of a new lung lesion and a splenic lesion; however, this was terminated due to poor toleration and disease progression into the spleen. Approximately 1 year after initial diagnosis the patient was admitted for hemoptysis and imaging studies revealed a 4.7 cm gastric fundus mass. He then underwent splenectomy, subtotal gastrectomy, distal pancreatectomy, and left lateral hepatic segmentectomy which revealed metastatic renal cell carcinoma measuring 13 cm in diameter with prominent areas of necrosis. The tumor invaded the submucosa of the stomach with ulceration and erosion of overlying mucosa, adhered to the liver, and invaded the spleen. Margins were negative for malignancy.
Case #3 is a 65-year-old Caucasian male that initially presented with a renal mass and concomitant gastric metastasis. The initial size of the renal mass is unclear; however, histopathological evaluation of the stomach body biopsies revealed ulceration of the mucosa and a histologic grade of 3. Immunochemistry evaluation revealed immunopositivity for CAM, CD10, RCC, vimentin, and EMA (focally positive). The metastatic tumor was negative for CEA, CDX2, CK7, CK20, CK5/6, and CD117. The overall histomorphology and immunophenotype was most consistent with metastatic renal cell carcinoma.
Case #4 is a 70-year-old Caucasian female that initially presented with clear cell renal cell carcinoma of the left kidney for which she underwent left radical nephrectomy. Histopathologic evaluation a maximum tumor dimension of 5 cm, histologic grade 2, no capsular invasion and margins negative for malignancy. She then presented with a mass in the right kidney and lung nodule for which she underwent partial right nephrectomy and right upper lobe resection by VATS procedure which confirmed recurrent and metastatic clear cell renal cell carcinoma. The patient completed 2 cycles of Sutent which was eventually changed to Pazopanib. She later developed liver lesions for which she underwent microwave ablation. Approximately six years after initial diagnosis the patient was found to have metastatic CCRCC with ulceration on gastric polyp biopsies with a recurrent gastric metastasis approximately 10 years after initial diagnosis.
Discussion
Metastatic tumors of the stomach are uncommon, although when observed the most frequent primary malignancies include lung cancer, breast cancer, and malignant melanoma [6,8]. Renal cell carcinoma (RCC) is a primary renal malignancy originating from the proximal tubular epithelium in the renal cortex. According to Bianchi et al., the most common sites of RCC metastases were lung (45%), bone (30%), lymph node (22%), liver (20%), adrenal (9%), and brain (8%). The rate of metastases in a single site was 61% versus 39% in two or more sites [2]. RCC metastasizing to the stomach is particularly rare. According to Mi-Young Kim et al., a systematic review of articles in English using a computerized search of PubMed database identified 36 patients with metastatic RCC to the stomach. Furthermore, they found that gastric metastases from RCC were more common in males than females (26 males vs. 9 females) and the median age at presentation was 67 years (range, 48-84 years) [5].
It is proposed that the biology of RCC, including histologic type, tumor heterogeneity, and biological mediators, may play a role in the metastatic patterns seen. The most common histologic subtype is clear cell which comprises 75-80% of cases. Of note, approximately 70% of patients with clear cell RCC have alterations in the Von-Hippel Lindau (VHL) gene. Furthermore, alterations in the mTOR, SETD2, PTEN, and KDM5c pathways were variable across different sites of metastases in a study from Gerlinger et al. A recent study from the Dana Farber Cancer Institute compared tissues derived from 53 primary RCC specimens and 73 corresponding metastases, which identified heterogenous expression of PD-L1 within lesions [3]. These findings suggest that the metastatic propensity of RCC is likely multifactorial, but also may have major implications for the therapy and management of metastatic RCC. However, the optimal treatment for gastric metastasis from RCC remains controversial.
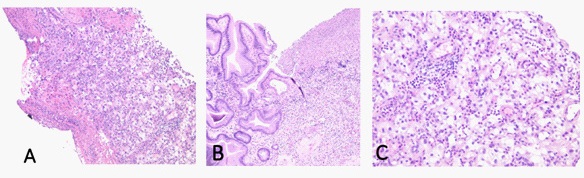
Generally speaking, the outcome of patients with RCC and gastric metastases is poor with a 5-year survival rate of approximately 12% [1]. According to Sakurai et al. prognostic factors in patients with gastric metastases from RCC include a grossly protruding gastric tumor, additional metastases to other organs, and development of gastric metastasis in <6.3 years from therapy [7]. Our investigation identified four RCC patients with gastric metastasis. M:F ratio was 2:2. The mean age was 58.3 years (range 45 to 70 years). Remarkably, all four cases were clear cell variant of RCC, and presented with gastric ulceration (See figure). The average time between initial diagnosis of RCC and gastric metastasis was 46.5 months. Three patients had previously underwent radical nephrectomy with no capsular invasion, and clear margins while one patient presented with renal mass and concomitant gastric metastasis. The mean initial tumor size was 8.73 cm. Fuhrman grading of II/IV, III/IV and IV/ IV were noted in one, two and one cases respectively. Among our four cases, the development of gastric metastasis was an average of 4 years from initial therapy. Additionally, three of the four patients also had RCC metastases to other organs, while one patient presented with concomitant gastric metastasis upon initially diagnosis of renal RCC.
Conclusion
Renal cell carcinoma comprises majority of primary renal malignancies; however, gastric metastasis is rare and often appears to be a late event in the course of RCC, with rare exceptions as in one of the cases presented above. Given the high mortality rate and variability in treatment options, considering metastatic RCC in differential diagnosis for a gastric lesion with ulceration is imperative in patients with atypical morphology or clinical presentation.
References
- Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, et al. “Distribution of metastatic sites in renal cell carcinoma: a populationbased analysis.” Annals of Oncology. 2012; 23: 973-980.
- Hakim C, Mendelson A, Patel J, Greer J,Sorser S. “Metastatic renal cell carcinoma presenting as gastrointestinal bleeding.” Case Reports in Gastroenterology. 2021; 15: 478-481.
- Pollheimer MJ, Hinterleitner TA, Pollheimer VS, Schlemmer A, Langner C, et al. “Renal cell carcinoma metastatic to the stomach: single-centre experience and literature review.” BJU International. 2008; 102: 315-319.
- Motzer RJ, Jonasch E, Michaelson MD, Nandagopal L, Gore JL, et al. Kidney Cancer, Version 2.2020 featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2019; 17: 1278-1285.
- Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. “Metastasis in renal cell carcinoma: biology and implications for therapy.” Asian Journal of Urology. 2016; 3: 286-292.
- Al Juboori A, Kaur S, Reddy A. “Metastatic renal cell carcinoma presenting as gastric ulcer: case report and literature review.” Case Reports in Gastrointestinal Medicine. 2017; 2017: 1-2.
- Kim M, Jung H, Choi KD, Song HJ, Lee JH, et al. Solitary synchronous metastatic gastric cancer arising from T1b renal cell carcinoma: a case report and systematic review. Gut and Liver. 2012; 6: 388-394.
- Sakurai K, Muguruma K, Yamazoe S, Kimura K, Toyokawa T, et al. Gastric metastasis from renal cell carcinoma with gastrointestinal bleeding: a case report and review of the literature.” International Surgery. 2014; 99: 86-90.