Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 2
High-grade thoracic sarcoma with HMBOX1-ALK fusion: Case report
Waldec Jorge David Filho1*; Angel Ayumi Tome Uchiyama1; Renan Ribeiro e Ribeiro2; Nídia Pinheiro Oliveira Silva3
1Department of Medical Oncology, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
2Department of Pathology, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
3Department of Radiology, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
*Corresponding Author : Waldec Jorge David Filho
Department of Medical Oncology, Hospital Alemão
Oswaldo Cruz, São Paulo, Brazil.
Email: waldecjorge@uol.com.br
Received : Dec 01, 2022
Accepted : Dec 21, 2022
Published : Dec 28, 2022
Archived : www.jjgastro.com
Copyright : © David Filho WJ (2022).
Abstract
Under the nomination of soft tissue sarcomas there are a large variety of mesenchymal neoplasms. This group of rare entities is responsible for just 1 to 2% of all kinds of cancer. Our purpose is to describe an even rarer subtype among these tumors. The patient of this report is a 28-year-old male who presented with pain in his left hemithorax and progressive fatigue. He had no previous comorbidities. An investigative MRI-scan showed a big and heterogeneous mass in his left hemithorax. A core-needle biopsy was performed and a diagnosis of high-grade spindle cell sarcoma, with no other specification, was rendered. Initially he underwent conventional treatments based upon surgery, chemotherapy and radiotherapy, but they were not enough to avoid progression of the disease. Before opting for a new agent, empirically, a next generation sequencing analysis was performed on the primary tumor tissue, to guide the subsequent therapy. An amazing pathogenic ALK fusion to HMBOX1, a new protein partner, was identified. Taking into consideration the genomic profiling of this tumor with a potential target therapy and some literature data of effectiveness of ALKi in sarcomas, we opted to start his treatment with crizotinib due to accessibility of this drug in our country, which can be released by the Brazilian health care insurance. He achieved a partial response with such a treatment, but unfortunately its duration was not very long. According to our knowledge, this is the first report of a high-grade sarcoma harboring an HMBOX1-ALK fusion. Further studies are necessary to determine if this tumor represents a novel entity or it belongs to a spectrum with other ALK-rearranged sarcomas. Until then, due to its aggressive clinical behavior and potential for targeted therapy, we propose the term “high-grade thoracic sarcoma with HMBOX1-ALK fusion”, for future references.
Keywords: Undifferentiated sarcoma; ALK fusion.
Abbreviations: STS: Soft Tissue Sarcomas; HMBOX1: Homeobox-Containing Protein1; ALK: Anaplastic Lymphoma Kinase; MRI: Magnetic Resonance Imaging; HPF: High-Power Fields; EMA: Epithelial Membrane Antigen; TLE1: Transducin-Like Enhancer Protein1; SMARCB1: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily B, Member1; SBRT: Stereotactic Body Radiation Therapy; TKI: Tyrosine Kinase Inhibitor; WHO: World Health Organization; NPM1: Nucleophosmin1; TPM: Tropomyosin3; IMT: Inflammatory Myofibroblastic Tumor; EML4: Echinoderm Microtubule Associated Protein Like4; NSCLC: Non-Small Cell Lung Cancer; VCL: Vinculin; ATP: Adenosine Triphosphate; ALKi: Anaplastic-Lymphoma Kinase Inhibitor; ORR: Overall Response Rate; CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progression of Disease; PSF: Progression-Free Survival; CT: Computed Tomography; NGS: Next-Generation Sequencing; FISH: Fluorescent In Situ Hybridization.
Citation: David Filho WJ, Uchiyama AAT, Ribeiro RR, Oliveira Silva NP. High-grade thoracic sarcoma with HMBOX1-ALK fusion: Case report. Japanese J Gastroenterol Res. 2022; 2(2): 1125.
Introduction
Soft Tissue Sarcomas (STS) enclose a large variety of mesenchymal neoplasms that can arise in any part of human body. This group of rare entities is responsible for just 1 to 2% of all kinds of cancer. Due to the great diversity of connective tissues, more than one hundred types and subtypes have been described so far. They are derived from primitive connective cells and there is a tendency to reproduce the tissue where the cell of origin is located in. However, there are many exceptions to this statement. For instance, an osteosarcoma can arise in soft tissue, a primary tongue synovial sarcoma has already been reported [1] and so on. Sometimes, it is not possible to identify, precisely, the subtype of STS we are dealing with. The malignant cells can assume an undifferentiated pattern, the majority of antigens are negative or helpless on immunohistochemistry and the final diagnosis cannot be established with certainty. In those cases, it is mandatory a molecular analysis, but such a methodology is not always routinely available in developing countries. Our purpose is to describe an unidentified “high-grade thoracic sarcoma with Homeobox-Containing Protein1- anaplastic lymphoma kinase fusion” (HMBOX1-ALK).
Case presentation
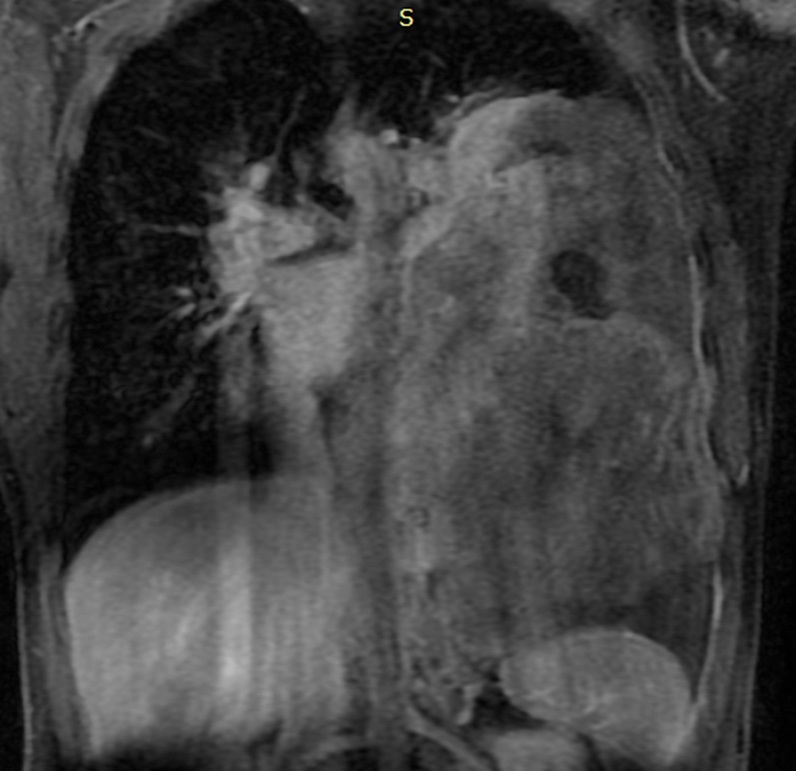
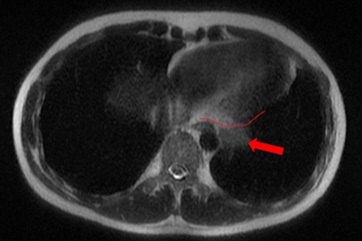
A 28-year-old male patient presented with onset of pain on his left hemithorax and progressive fatigue, in September, 2020. He had no previous comorbidities. An investigative MRI (magnetic resonance imaging) scan showed a large and heterogeneous mass on his left hemithorax with partial lung atelectasis, inferior displacement of diaphragm, mediastinal shift to the right and small pleural effusion (Figures 1a, 1b and 1c). The tumor was in contact with the inferior esophagus and the descending aorta. The thoracic wall (muscle and ribs) was not compromised. These findings suggested, possibly, a mass of pleural origin. An initial core-needle biopsy was performed in October, 2020 to clarify the diagnosis.
Microscopic examination of the sample mass showed a highly cellular spindle cell proliferation with scant cytoplasm and focal fibrous stroma, which was favored to represent a cellular solitary fibrous tumor or monophasic synovial sarcoma on morphologic examination. Immunohistochemical or molecular analysis of the biopsy sample was not performed at that occasion. Subsequently, the patient underwent a R1 resection of the visceral pleural lesion. Gross examination of the specimen revealed a roughly oval shaped nodular lesion, weighting 2,840 Kg and measuring 19.3 cm x 18.5 cm x 9.3 cm, with a white and solid cut surface, along with multiple irregular hemorrhagic-tan colored fragments measuring altogether 20.0 cm x 19.0 cm x 2.5 cm. Microscopic examination revealed a highly cellular mesenchymal neoplasm with small spindle cells forming long fascicles. Focal fibrous or myxoid change was seen. Neoplastic cells had scant cytoplasm and monomorphic mildly atypical nuclei. Scattered foci of osteochondral differentiation were present. Mitotic activity was relatively high and ranged from 7 mitotic figures per 10 high-power fields (HPF) up to 17 per 10 HPF. Necrosis was seen in up to 30% of tumor volume, as well as hemorrhagic areas with a degenerative aspect. By immunohistochemistry, tumor cells were negative for cytokeratins AE1/AE3, smooth muscle actin, CD34, desmin, epithelial membrane antigen (EMA), S100, and transducin-like enhancer protein 1 (TLE1). Intact immunohistochemical expression of INI1 (SMARCB1) and H3K27me3 were seen. A diagnosis of high-grade spindle cell sarcoma with no other specification was rendered, and a molecular testing was recommended.
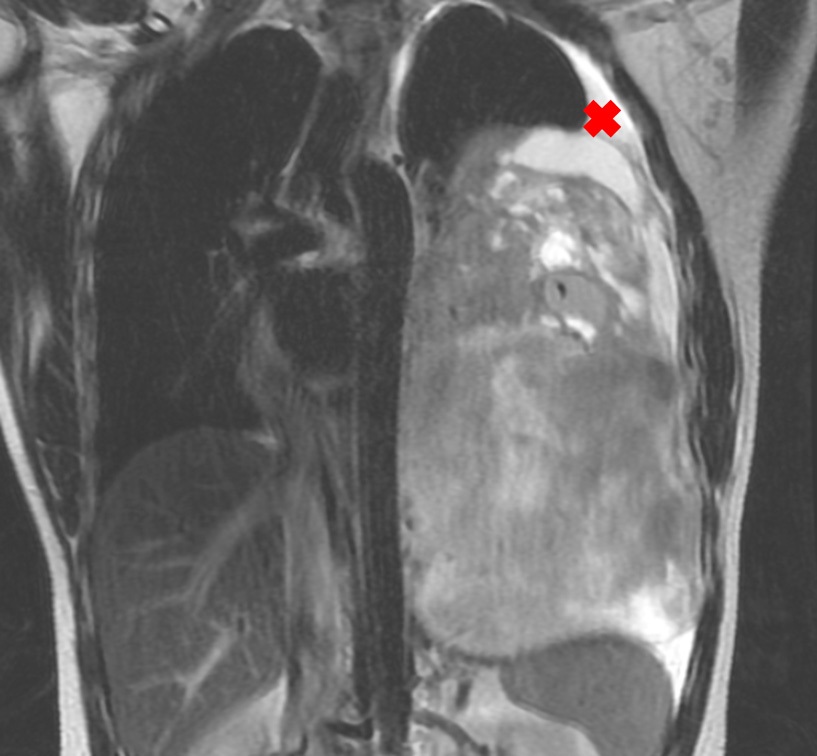
After the surgery, evaluation images in February, 2021 showed residual mass close to the descending aorta, the distal esophagus and the pericardial leaflet (Figure 2).
Then, the patient received 5 cycles of cytostatic treatment with the combination of ifosfamide and doxorubicin, presumably to prolong the progression-free survival, from November 2020, to March, 2021.
Right after the chemotherapy, he also received Stereotactic Body Radiation Therapy (SBRT) - 35 Grays in 5 sessions in June, 2021), as well as, to decrease the likelihood of disease increment. In vain, so the persistence of disease in contact with the pericardium, as showed in Figure 3, would progress shortly
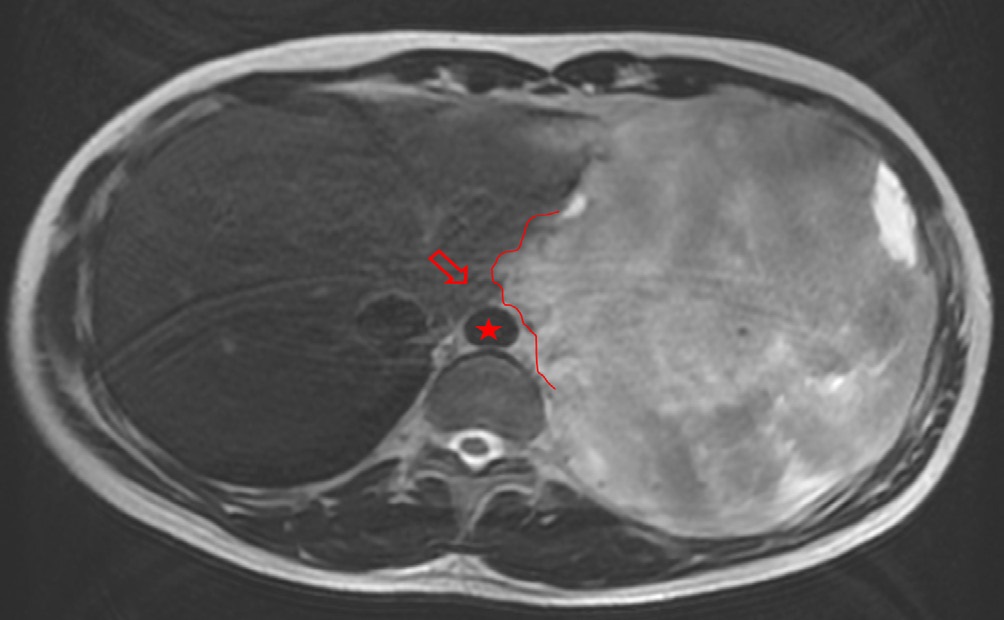
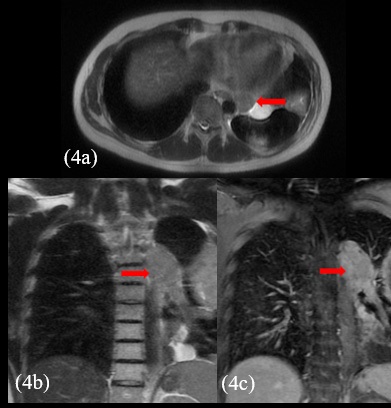
The patient presented with voluminous and symptomatic disease less than six months of chemotherapy end and less than two months of radiotherapy. He restarted with pain in left hemithorax, cough, shortness of breath, lack of appetite and daily vespertine fever. MRI scans in August, 2021 showed new lesions on pleural surfaces beyond the residual mass previously seen (Figures 4a, 4b and 4c). One of the lesions, on the extra-pleural fat adjacent to the diaphragm, extended inferiorly, pushing downward the muscle.
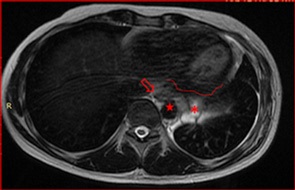
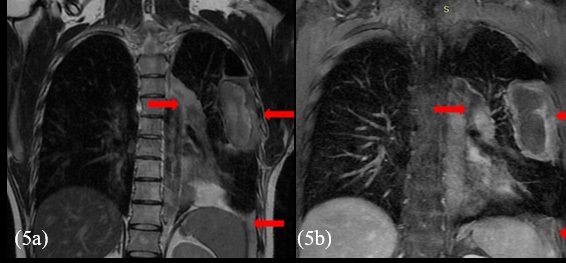
Before opting for some new agent, empirically, a next generation sequencing analysis was performed on the primary tumor tissue, to guide the subsequent therapy. An amazing pathogenic HMBOX1-ALK gene fusion was identified. Thus, we decide to continue his treatment by administering crizotinib, a tyrosine -kinase inhibitor (TKI) approved in Brazil for usage in lung cancer with ALK translocation. At the beginning of oral therapy, he manifested nausea grade 2, vomit grade 1 and diarrhea grade 2, according to WHO (World Healthy Organization) profile toxicity; all of these symptoms were manageable with regular antiemetic drugs and loperamide. Approximately, one month after the beginning of the new treatment, he reported reduction of chest pain and cough, as well as diminishing of tiredness and dyspnea, which were present only with great effort. The evaluation images with 45 days of crizotinib therapy showed reduction of all the lesions with appearance of necrotic/hemorrhagic areas (Figures 5a and 5b).
In January, 2022, the patient started, once more, by complaining of pain in left hemithorax, cough and shortness of breath, lack of appetite and daily vespertine fever. A little longer he was hospitalized and ended up dying due to progression of disease.
Discussion
Identification of molecular targets with potential of driving oncogenesis remains a cornerstone for synthesizing new selective cancer therapies. ALK is a receptor tyrosine kinase which has been reported to be a driver in oncogenic pathways in a variety of cancer subtypes, as a result of its fusion to several partner genes, such as nucleophosmin 1 (NPM1) in anaplastic large cell lymphoma, to tropomyosin 3 (TPM3) or TPM4 in inflammatory myofibroblastic tumor (IMT), to echinoderm microtubule associated protein like 4 (EML4) in Non-Small Cell Lung Carcinoma (NSCLC), and to vinculin (VCL) in renal medullary carcinoma. The ALK oncogenic pathway can also be activated as a result of missense mutations in neuroblastoma and anaplastic thyroid cancer [2]. In this clinical case we report a novel ALK fusion partner HMBOX1 in a high-grade sarcoma. Given that all ALK fusion kinases retain an intact adenosine triphosphate (ATP)-binding pocket (where most ALK inhibitors bind), it is likely that ALK inhibitors (ALKi) could be effective against any tumor type that harbors such an ALK fusion [3-6]. Regarding solid tumors, crizotinib and newer generations ones, such as alectinib, ceritinib, brigatinib, and lorlatinib are approved for treatment of patients with ALK fusion–positive advanced NSCLC, given the superior outcomes achieved by these agents when compared with standard chemotherapy [7,8]. However, data about the activity and efficacy of ALKi in ALK fusion–positive cancers other than non-NSCLC are scarce.
Anyways, previous studies have shown that crizotinib is effective and achieves a durable response in ALK-positive tumors other than NSCLC. In these studies, crizotinib resulted in overall response rate (ORR) of 66.7% to 86.0% in patients with IMT and 11.8% in patients with other solid tumors excluding NSCLC, with a 2-year progression-free survival (PSF) of 63% for lymphoma and 67% for IMT [9,10]. A more recent publication showed similar results, and it also demonstrated the effectiveness of alectinib in this scenario with an ORR for the initial ALK-TKI therapy of 85.7% (95% CI, 44 to 97), including two patients who received alectinib and achieved Complete Response (CR). The median progression-free survival was 8.1 months [11]. Additionally, a case series of ALK fusion–positive gastrointestinal cancers showed that from twelve evaluable patients, 41% achieved a Partial Response (PR) to first-line ALKi treatment, whereas no CR was registered. The ORR was 41% and stable disease (SD) was achieved by five patients (41%), with a disease control rate of 82%. The remaining two patients (15%) had progression of disease (PD) at the first evaluation. Some patients (38%) were able to receive a second line of ALKi therapy after disease progression, with further clinical benefit [6].
ALK-positive sarcoma is predominantly seen in pediatric or middle-aged patients, with ALK fusions identified in about 50% of IMT cases, 3.6% of sarcomatous malignancies, and it is extremely rare in rhabdomyosarcoma and leiomyosarcoma [12]. Wu et al. reviewed 33 cases of sarcoma and sarcomatous malignancies harboring ALK fusions that were treated with crizotinib and showed objective response rate of 86.7% and disease control of 96.7%. This study also reported a rapid improvement of symptoms, within one to two weeks in some cases, including patients with extensive diseases and poor performance status [13].
Our patient presented with a rapidly progressive pleural tumor, refractory to standard chemo-radiation therapy and harboring an ALK fusion to HMBOX1, a new protein partner. Taking into consideration the genomic profiling of this tumor with a potential target therapy and some literature data of effectiveness of ALKi in sarcomas, we opted to start his treatment with crizotinib due to accessibility of this drug, that can be released by the Brazilian health care insurance. He experienced a significant amelioration of his symptoms in a couple of weeks. Besides, he achieved a partial response in about six weeks of treatment, according to the CT-scan images. The duration of response was short and his disease progressed after four months of therapy. Unfortunately, there was not enough time to prescribe him a second-generation ALKi.
Cell clones carrying ALK resistance mutations may explain the modest benefit with first-generation ALKi. High-grade sarcomas harboring ALK fusion are rare, thus we must understand better the roll of this tyrosine kinase receptor as an oncogenic driver in soft tissue tumors and the association with this new protein fusion partner HMBOX1. Nevertheless, the patient benefited from the treatment, with a PSF of four months.
HMBOX1-ALK fusions appear to be a rare phenomenon, and have been previously described in three separate studies. In the study conducted by Liu et al, a single tumor harbored an HMBOX1-ALK fusion among 44 ALK-positive NSCLC, whose tumors underwent genomic characterization [14]. A metastatic gastric adenocarcinoma reported by Ambrosini et al. was also found to harbor an HMBOX1-ALK fusion by Next-Generation Sequencing; (NGS) and Fluorescent In Situ Hybridization (FISH) [15]. In 2021, Dermawan et al. reported six cases of a novel cutaneous tumor named “superficial ALK-rearranged myxoid spindle cell neoplasm”; one of them harbored HMBOX1-ALK fusion detected by NGS [16]. All of them were localized in the dermis or superficial subcutis and showed bland morphologic features with low mitotic activity. So far, the authors reported no recurrence at all. Despite the molecular similarity, it is unlikely the tumor we have described represents the same neoplasm, mainly taking into account the significant differences in clinicopathologic presentation.
Conclusion
Our patient presented with a pleural mass with aggressive clinical behavior and histopathologic features consistent with a high-grade sarcoma. Furthermore, by immunohistochemistry analysis, most tumors in the aforementioned study co-expressed CD34 and S100, while our example is negative for both markers. To the best of our knowledge, this is the first report of a high-grade sarcoma harboring an HMBOX1-ALK fusion. Further studies are necessary to determine whether this tumor represents a novel entity or belongs to a spectrum with other ALK-rearranged sarcomas. Until then, due to its aggressive clinical behavior and potential for targeted therapy, we propose the term “high-grade thoracic sarcoma with HMBOX1-ALK fusion”, for future references.
Declarations
Acknowledgment: The authors are grateful to the patient’s family who permitted the publication of his case.
References
- Bridge JA, Bridge RS, Borek DA, Shaffer B, Norris CW. Translocation t(X; 18) in orofacial synovial sarcoma. Cancer. 1988; 62: 935- 937.
- Mano H. ALKoma: A Cancer Subtype with a Shared Target. Cancer Discov. 2012; 2: 495-502.
- Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, et al. Crizotinib in ALK-Rearranged Inflammatory Myofibroblastic Tumor. N Engl J Med. 2010; 363: 1727-1733.
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N Engl J Med. 2010; 363: 1693-1703.
- McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, et al. Genomic Alterations of Anaplastic Lymphoma Kinase May Sensitize Tumors to Anaplastic Lymphoma Kinase Inhibitors. Cancer Res. 2008; 68: 3389-3395.
- Ambrosini M, Del Re M, Manca P, Hendifar A, Drilon A, et al. ALK Inhibitors in Patients With ALK Fusion–Positive GI Cancers: An International Data Set and a Molecular Case Series. JCO Precis Oncol. 2022; 6: e2200015.
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Cancer Netw JNCCN. 2021; 19: 254-266.
- Clinical Practice Living Guidelines - Metastatic Non-Small-Cell Lung Cancer | ESMO. 2022.
- Gambacorti-Passerini C, Orlov S, Zhang L, Braiteh F, Huang H, et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am J Hematol. 2018; 93: 607-614.
- Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J Clin Oncol Off J Am Soc Clin Oncol. 2017; 35: 3215-3221.
- Takeyasu Y, Okuma HS, Kojima Y, Nishikawa T, Tanioka M, et al. Impact of ALK Inhibitors in Patients With ALK-Rearranged Nonlung Solid Tumors. JCO Precis Oncol. 2021; 5: PO.20.00383.
- Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, et al. Crizotinib in ALK+ inflammatory myofibroblastic tumors-Current experience and future perspectives. Pediatr Blood Cancer. 2018; 65.
- Wu J, Hu Y, Abdihamid O, Huang G, Xiao S, et al. Crizotinib in Sarcomatous Malignancies Harboring ALK Fusion With a Definitive Partner(s): Response and Efficacy. Front Oncol. 2021; 11: 684865.
- Liu S, Huang T, Liu M, He W, Zhao YS, et al. The Genomic Characteristics of ALK Fusion Positive Tumors in Chinese NSCLC Patients. Front Oncol. 2020; 10:726.
- . Ambrosini M, Del Re M, Manca P, Hendifar A, Drilon A, et al. ALK Inhibitors in Patients With ALK Fusion-Positive GI Cancers: An International Data Set and a Molecular Case Series. JCO Precis Oncol. 2022; 6: e2200015.
- Dermawan JK, Azzato EM, Goldblum JR, Rubin BP, Billings SD, et al. Superficial ALK-rearranged myxoid spindle cell neoplasm: a cutaneous soft tissue tumor with distinctive morphology and immunophenotypic profile. Mod Pathol. 2021; 34: 1710-1718.