Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
The clinical value of serum AFP, AFP-L3 and DCP alone and in combination in the diagnosis of primary liver cancer
Huihui Mao; Mengxing Dong; Hui Zhao; Nana Dong; Hanxiao Ren*
Laboratory Medical Center of the Second Hospital of Shandong University, Beiyuan Street, Jinan City, Shandong Province, China.
*Corresponding Author : Hanxiao Ren
Laboratory Medical Center of the Second Hospital
of Shandong University, No247, Beiyuan Street,
Jinan City, Shandong Province, China.
Email: hanxiao_ren@163.com
Received : Oct 28, 2022
Accepted : Nov 21, 2022
Published : Nov 25, 2022
Archived : www.jjgastro.com
Copyright : © Ren H (2022).
Abstract
Objective: To analyze the diagnostic value of AFP, AFP-L3 and DCP in primary liver cancer.
Methods: Serum samples were collected from outpatients, inpatients and healthy people who visited the Second Hospital of Shandong University. The contents of AFP, AFP-L3 and DCP in serum samples were detected by automatic chemiluminescence immunoassay. According to the research purpose, they were divided into 5 groups: chronic hepatitis B group, hepatitis B cirrhosis group, other etiology (alcoholic and hepatitis C) cirrhosis group, primary liver cancer group and healthy control group. The receiver operator characteristic curve (ROC curve) was drawn to evaluate the diagnostic performance of the three markers alone and in combination in detecting primary liver cancer.
Results: Compared with the other 4 groups, the serum AFP, AFP-L3 and DCP levels in the primary liver cancer group were significantly increased, with statistical significance. The results of ROC analysis showed that the combined detection of AFP, AFP-L3 and DCP had the largest AUC area (0.852), followed by DCP (AUC of 0.794), while AFP (AUC of 0.734) and AFP-L3 (AUC of 0.726) were more small.
Conclusion: The combined diagnostic efficiency of AFP, AFP-L3 and DCP is significantly higher than that of single detection, which significantly improves the diagnosis rate of liver cancer and is worthy of clinical promotion.
Keywords: Primary liver cancer; Alpha fetoprotein; AFP-L3; Des-gamma-carboxy prothrombin.
Citation: Mao H, Dong M, Zhao H, Dong N, Ren H. The clinical value of serum AFP, AFP-L3 and DCP alone and in combination in the diagnosis of primary liver cancer. Japanese J Gastroenterol Res. 2022; 2(15): 1121.
Introduction
Primary Liver Cancer (PLC) is one of the most common malignant tumors. According to the data of GLOBOCAN 2020, there are 906,000 new cases and 830,000 deaths of PLC worldwide, ranking 6th and 3th in malignant tumors respectively, mainly in Asia. Among them, the incidence of PLC in China ranks the 5th in malignant tumors, with 410,000 new cases, accounting for about half of the new cases worldwide. The mortality rate of PLC ranks 2th in malignant tumors, with 391,000 deaths [1]. PLC seriously threatens the life and health of our people. Due to the development of imaging technology in recently years, such as Conventional abdominal ultrasound, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), etc., the detection rate of early carcinoma of liver has been significantly improved [2]. However, it is not convincing to rely solely on imaging examination to diagnose liver cancer. Even though detection rate of liver cancer can be further enhanced by combing with Alpha Fetoprotein (AFP), the most widely used serum marker related to liver cancer in clinically, there are still 30%~40% patients with liver cancer who do not have elevated AFP while some patients with benign liver diseases, such as chronic hepatitis and cirrhosis, have rising AFP level. Some new liver cancer markers, such as AFP-L3 and Des-Gamma-Carboxy Prothrombin (DCP), have also attracted increasing clinical attention [3]. Therefore, the aim of this research is to analyze the diagnostic value of AFP, AFP-L3 and DCP in liver cancer.
Patients and methods
Study participants
Serum samples were collected from outpatients, inpatients and healthy people who visited the Second Hospital of Shandong University from January 2021 to December 2021. All subjects were collected consecutively, totally 743 cases aged 28-77 years, including 530 males and 213 females. Relying on research purpose, they were divided into chronic hepatitis B group (215 cases), hepatitis B cirrhosis group (245 cases), other causes (alcohol and hepatitis C) cirrhosis group (130 cases), primary liver cancer group (62 cases) and healthy control group (91 cases).
Inclusion and exclusion criteria
Inclusion criteria: 1. The subjects who had complete and accurate information and clinical data were detected for AFP, AFP-L3 and DCP; 2. All subjects had definite diagnosis results. Guideline for primary care of chronic hepatitis B (2020), Guidelines for Diagnosis and treatment of Liver Cirrhosis (2019 ) and Guidelines for Diagnosis and Treatment of Primary Liver Cancer (2022) were used as diagnostic criteria of chronic hepatitis B, liver cirrhosis and PLC, respectively; 3. The subjects conformed the informed consent principle and signed informed consent form. Exclusion criteria: 1. The people with incomplete research data; 2. The people with confirmed malignant tumors of stomach, intestine, kidney and other organs; 3. The people who has recently taken warfarin anticoagulants, vitamin K, or vitamin K antagonists; 4. The people with severe cardiac insufficiency, renal insufficiency, and water-electrolyte disorder; 5. The people who are pregnant or breastfeeding and the people with severe malnutrition.
Sample collection and methods
The serum was taken from 5 mL fasting venous blood of the patient after centrifuging at 3500 r/min for 10 mins. The contents of AFP, AFP-L3 and DCP in serum samples were detected by Hotgen Biotech C2000 automatic chemiluminescence immunoassay. The reagents, control materials and calibration materials were from original reagents of Hotgen Biotech company. All the results were obtained under the qualified condition of the instrument operation and quality control. In this research, 20 ng/ml, 1 ng/ml and 40 ng/ml were used as the cut-off values of AFP, AFP-L3 and DCP respectively. The detection results of AFP, AFP-L3 and DCP above the cut-off values were defined as positive.
Statistical analysis
SPASS 25.0 statistical software was used for statistical analysis. The Shapiro-Wilk test (S-W) was used to test the normality of each group’s data. The experimental data was expressed as quartiles [P50(P25,P75)] when it did not meet the normal distribution. Kruskal-wallis H test was used for data comparison among multiple groups and Chi-square test was used for enumeration data, expressed as (n, %). The Receiver Operator Characteristic Curve (ROC Curve) was drawn, and the area under the ROC-AUC was calculated to evaluate the diagnostic value of each index. For all test results, P < 0.05 was considered statistically significant.
Results
Basic information
According to research purpose, the subjects were divided into 5 groups: chronic hepatitis B group, hepatitis B cirrhosis group, other causes (alcohol and hepatitis C) cirrhosis group, primary liver cancer group and healthy control group. The age composition of each group was not normal distribution. There were no significant differences and statistically significant in age and gender among all groups (P > 0.05). As shown in Table 1.
Table 1: Comparison of age and gender in each group.
Category |
The number of cases |
Age |
Gender |
|
Female |
Male |
|||
chronic hepatitis B group |
215 |
51.00(48.00,56.00) |
67(31.46%) |
148(27.92%) |
hepatitis B cirrhosis group |
245 |
50.00(43.50,57.00) |
68(31.92%) |
177(33.40%) |
other causes cirrhosis group |
130 |
51.00(46.00,57.00) |
41(19.25%) |
89(16.79%) |
primary liver cancer group |
62 |
54.00(47.00,59.25) |
11(5.16%) |
51(9.62%) |
healthy control group |
91 |
48.00(42.00,57.00) |
26(12.21%) |
65(12.26%) |
Z/x² |
6.908 |
4.898 |
||
P |
0.141 |
0.298 |
||
Comparison of serum AFP, AFP-L3 and DCP in each group
There were significant differences in serum AFP, AFP-L3 and DCP levels among chronic hepatitis B group, hepatitis B cirrhosis group, other causes cirrhosis group, primary liver cancer group and healthy control group (P < 0.05). In addition, the results indicated that the levels of serum AFP, AFP-L3 and DCP in PLC group were significantly higher than those in other groups. As shown in Table 2.
Table 2: Comparison of serum markers in each group.
Category |
The number of cases |
AFP |
AFP-L3 |
DCP |
chronic hepatitis B group |
215 |
2.79(2.09,4.06)* |
0.60(0.60,0.60)* |
9.19(5.60,16.13)*# |
hepatitis B cirrhosis group |
245 |
2.42(1.59,4.08)* |
0.60(0.60,0.60)* |
11.39(6.06,18.95)*# |
other causes cirrhosis group |
130 |
2.99(1.94,5.22)* |
0.60(0.60,0.60)* |
15.88(8.66,24.46)* |
primary liver cancer group |
62 |
7.80(2.80,223.59) |
0.60(0.60,27.24) |
35.69(12.17,183.35) |
healthy control group |
91 |
2.98(2.27,4.07)* |
0.60(0.60,0.60)* |
9.58(5.22,14.34)*# |
Z |
47.662 |
120.018 |
73.771 |
|
P |
0.000 |
0.000 |
0.000 |
Note:“*”indicates P < 0.05 compared with liver cancer group;“#” indicates P < 0.05 compared liver cirrhosis group.
ROC curve analysis of serum AFP, AFP-L3 and DCP individual and combined detection for primary liver cancer
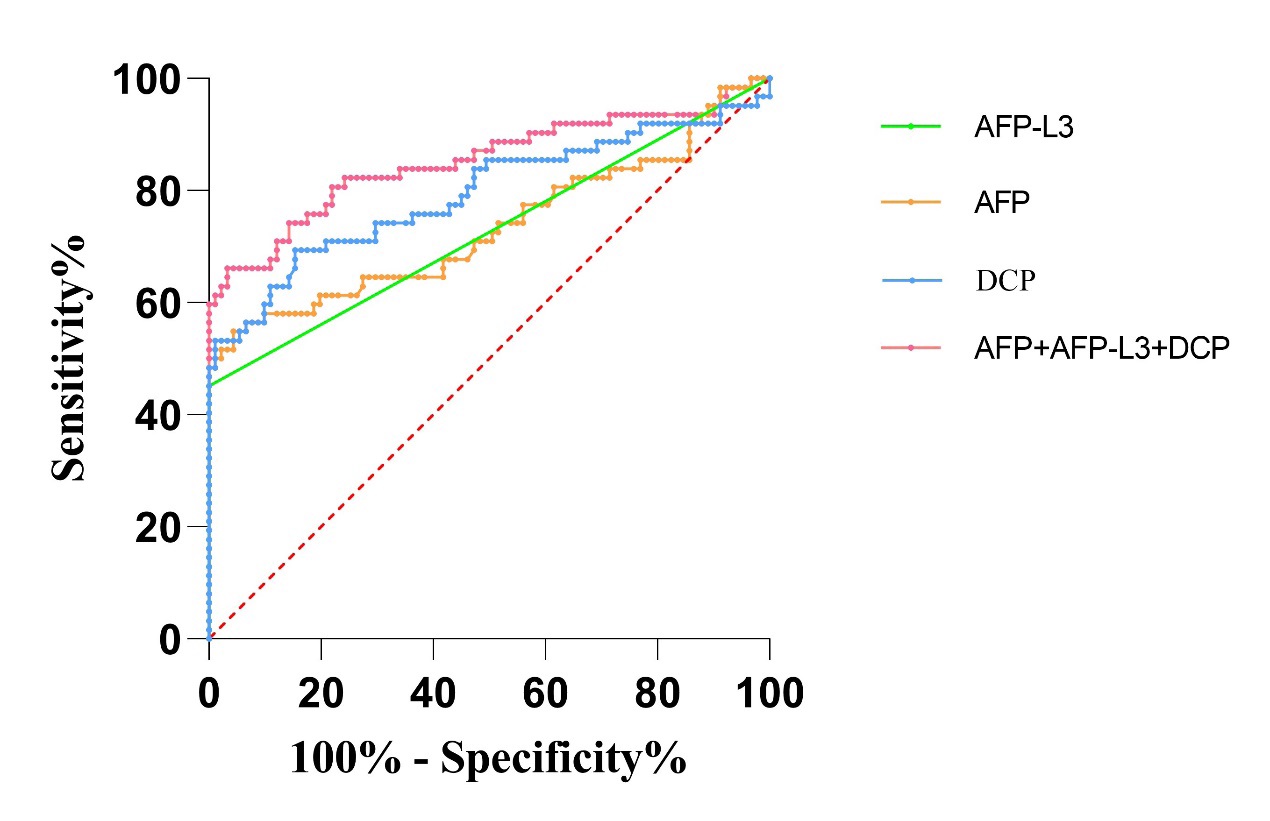
The results of ROC curve analysis demonstrated that the AUC of AFP, AFP-L3, DCP were 0.7342 (95%CI:0.6446~0.8239), 0.7258 (95%CI:0.6377~0.8140), 0.7941 (95%CI:0.7135~0.8748), respectively. The AUC of AFP, AFP-L3 and DCP combined detection was 0.8522 (95%CI: 0.7826-0.9218), revealing that the diagnostic efficiency of the three combined detection was significantly higher than single detection (P < 0.05), shown in Table 3 and Figure 1.
Table 3: Comparison of ROC curves in serum markers individual and combined detection for primary liver cancer.
Serum markers |
Area under curve (AUC) |
Standard error (SE) |
P value |
95%CI |
AFP |
0.7342 |
0.04573 |
<0.0001 |
0.6446~0.8239 |
AFP-L3 |
0.7258 |
0.04498 |
<0.0001 |
0.6377~0.8140 |
DCP |
0.7941 |
0.04115 |
<0.0001 |
0.7135~0.8748 |
AFP+AFP-L3+DCP |
0.8522 |
0.03552 |
<0.0001 |
0.7826~0.9218 |
Discussion
Primary liver cancer is the common malignant tumor and cause of tumor-related death in China. Although the standardized incidence and mortality of liver cancer population in China have demonstrated a downward trend in recent years, burden of PLC is still serious due to the fact that huge population base, aging, etc [3-5]. Therefore, standardized screening and monitoring is key to early detection, early diagnosis and radical cure of liver cancer.
AFP is the most widely applicated serum marker of liver cancer currently [6]. However, with the rapid development of medical imaging, the diagnostic proportion of small liver cancer keeps increasing while the specificity and sensitivity of AFP gradually keeps decreasing [7]. In 2011, American Association for the Study of Liver Diseases suggested that AFP should no longer be used as a screening indicator for liver cancer [8,9]. Due to the fact that chronic hepatitis B infection is the main reason causing liver cancer in China [10], AFP detection can still be used as an important method for early screening of liver cancer. AFP-L3, as a heterogeneity of AFP, is mainly derived from a glycoprotein of liver cancer cells [11,12]. The research showed that there has no correlation between AFP-L3 and AFP and the former can be used as an independent diagnostic indicator for liver cancer. DCP is Des-γ-carboxy-prothrombin, also known as PIVKA-II, which is an abnormal form of prothrombin [13,14]. Serum DCP is significantly increased in patients with liver cancer, suggesting that it may be a new tumor biomarker to diagnose liver cancer [15]. Therefore, this research detected the levels of serum markers AFP, AFP-L3 and DCP in chronic hepatitis B group, hepatitis B cirrhosis group, other causes cirrhosis group, primary liver cancer group and healthy control group and further explored their value in the early diagnosis of liver cancer.
The results of this study showed that serum AFP, AFP-L3 and DCP from primary liver cancer group were remarkably higher than those from chronic hepatitis B group, hepatitis B cirrhosis group, other causes cirrhosis group and healthy control group, and these differences were statistically significant (P < 0.05), indicating the AFP, AFP-L3 and DCP can be used as serological indicators to diagnose primary liver cancer.
Further, ROC curve analysis of this research showed that AUC of AFP was 0.7342 (95%CI: 0.6446-0.8239) and AUC of AFP-L3 was 0.7258 (95%CI: 0.6377-0.8140). Both of them were significantly lower than AUC of DCP with 0.7941 (95%CI: 0.7135-0.8748), illuminating that serum DCP has prominent diagnostic efficiency in the laboratory diagnosis of liver cancer. A large number of previous studies have shown that even if there was no correlation between AFP, AFP-L3 and DCP, but the combined detection of their also have complementary to diagnose liver cancer. The sensitivity and specificity from combined diagnosis were higher than the results of single diagnosis. This research strongly supported the view by the fact that the AUC of AFP, AFP-L3 and DCP combined diagnosis was 0.8522 (95%CI: 0.7826-0.9218), which was significantly higher than AUC of AFP,AFP-L3 and DCP individual diagnosis. This provided the evidence for combined detection of AFP, AFP-L3 and DCP to improve the diagnostic efficiency of liver cancer.
Conclusion
In conclusion, although the individual detection of AFP, AFP-L3 and DCP has certain value for liver cancer diagnosis, there are still some limitations in the differential and diagnosis for diseases and a huge gap with ideal diagnostic goal. The combined detection of AFP, AFP-L3 and DCP can considerably improve the diagnostic efficiency of liver cancer to avoid misdiagnosis and missed diagnosis, applying a better diagnostic indicator for clinical popularization and application.
Declarations
Acknowledgement: The authors would like to thank Laboratory Medical Center of The Second Hospital of Shandong University, for their support.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: This study was approved by the Ethics Committee of The Second Hospital of Shandong University (KYLL-2021(LW)088).
Research funding: This work was supported by the Natural Science Foundation of Shandong Province (ZR2020QH278).
References
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, et al. Cancer statistics for the year 2020: An overview. International Journal of Cancer. 2021; 149: 778-789.
- Nan Y, Xu X, Gao Y, Wang R, Li W, et al. Consensus on the secondary prevention of primary liver cancer. Hepatol Int. 2021; 15:1289-1300.
- Guideline for stratified screening and surveillance of primary liver cancer (2020 Edition). Zhonghua Gan Zang Bing Za Zhi. 2021; 29: 25-40.
- Shi TT, Liu ZQ, Fan H, Zhang PY, Yu SZ, et al. Analysis on incidence trend of liver cancer in China, 2005-2016. Zhonghua Liu Xing Bing Xue Za Zhi 2022; 43: 330-335.
- Shi JF, Cao M, Wang Y, Bai F-Z, Lei L, et al. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021; 148: 1051-1065.
- Zheng Y, Zhu M, Li MA-O. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020; 146: 2439-2446.
- Wong GL, Chan Hl Fau - Tse Y-K, Tse Yk Fau - Chan H-Y, Tse C-H, O S Lo A, et al. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014; 59: 986-995.
- Ding WX, Wang H, Zhang Y. Recent insights into the pathogeneses and therapeutic targets of liver diseases: Summary of the 4th Chinese American Liver Society/Society of Chinese Bioscientists in America Hepatology Division Symposium in 2021. Liver Res. 2022; 6: 50-57.
- Zhao M, Pan X, Yin Y, Hu H, Wei J, et al. Cost-Effectiveness Analysis of Five Systemic Treatments for Unresectable Hepatocellular Carcinoma in China: An Economic Evaluation Based on Network Meta-Analysis. Front Public Health. 2022; 10: 869960.
- Xu XF, Liang L, Xing H, Shen F, Huang DS, et al. Clinical utility of serum biomarkers for hepatocellular carcinoma. Biomark Med. 2021; 15: 151-155.
- Kawahara I, Fukuzawa H, Urushihara N, Kosaka Y, Kuroda Y, et al. AFP-L3 as a Prognostic Predictor of Recurrence in Hepatoblastoma: A Pilot Study. J Pediatr Hematol Oncol. 2021; 43: e76-e79.
- Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2021; 100: e27673.
- Su TH, Peng CY, Chang SH, Tseng T-C, Liu C-J, et al. Serum PIVKA-II and alpha-fetoprotein at virological remission predicts hepatocellular carcinoma in chronic hepatitis B related cirrhosis. J Formos Med Assoc. 2021; S0929-6646: 00361-00362.
- Feng H, Li B, Li Z, Wei Q, Ren L, et al. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021; 21: 401.
- Ahn KS, O’Brien DR, Kim YH, Kim T-S,Yamada H, et al. Associations of Serum Tumor Biomarkers with Integrated Genomic and Clinical Characteristics of Hepatocellular Carcinoma. Liver Cancer. 2021; 10: 593-605.