Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 2
Challenges in diagnosing acute mesenteric ischemia in a patient with COVID 19
Thilini Sudusinghe1; Adel Ekladious1,2*; Jennifer J Shoobridge3
1Department of General Medicine, Royal Hobart Hospital, Hobart, Tasmania.
2Faculty of Health and Medical Sciences, University of Western Australia, Perth, Western Australia.
3Department of Radiology, Royal Hobart Hospital, Hobart, Tasmania.
*Corresponding Author : Adel Ekladious
Faculty of Health and Medical Sciences, University of
Western Australia, Perth, Western Australia.
Email: adel.ekladious@ths.tas.gov.au
Received : Oct 12, 2022
Accepted : Oct 27, 2022
Published : Nov 02, 2022
Archived : www.jjgastro.com
Copyright : © Ekladious A (2022).
Abstract
Extrapulmonary manifestations of COVID-19 including acute mesenteric ischemia are being increasingly recognized and associated with extremely poorer clinical outcomes. Because of unclear clinical presentation, non-specific early imaging, and laboratory findings, may lead to loss of valuable time. Considering the high thrombotic risk in COVID-19, it is crucial to consider the high suspicion of acute mesenteric ischemia in patients with acute abdominal pain. Here, we present a challenging case of an elderly female who developed an acute superior mesenteric artery thrombosis as a result of COVID 19.
Keywords: Challenges; COVID-19; Acute mesenteric ischemia.
Abbreviations: AMI: Acute Mesenteric Ischemia; NOMI: Non-Occlusive Mesenteric Ischemia; MVT: Mesenteric Venous Thrombosis; AMAT: Acute Mesenteric Artery Thrombosis; AMAE: Acute Mesenteric Artery Embolism; SMA: Superior Mesenteric Artery; CTA: Computed Tomography Angiogram; CRC: Reactive Protein; WCC: White Cell Count.
Citation: Sudusinghe T, Ekladious A, Shoobridge JJ. Challenges in diagnosing acute mesenteric ischemia in a patient with COVID 19. Japanese J Gastroenterol Res. 2022; 2(14): 1117.
Introduction
The mortality and morbidity associated with COVID-19 have raised increasing concerns around the world. Although efforts to recognize and manage COVID-19 have been focused on evaluating the respiratory complications, it has now become aware that COVID-19 infection occasionally involves atypical presentations, such as gastrointestinal manifestations and thrombotic complications [1]. Many Studies have reported that patients infected with COVID 19 are prone to coagulopathy, however, the exact mechanism for the thrombosis is yet to be clarified [2]. Acute mesenteric ischemia, especially superior mesenteric artery (SMA) occlusion, is a critical condition with a high mortality rate of 60–80% which requires urgent diagnosis and treatment [1]. Despite growing concerns about these life-threatening thrombotic complications, early diagnosis is challenging because of variable symptoms, non-specific laboratory investigations, and nonspecific radiological findings. Early diagnosis is necessary to commence appropriate treatment, whereas diagnostic delay contributes to increased morbidity and mortality. In this case, we depict the abdominal CT findings of an 82 year-old female patient with COVID-19 who developed severe abdominal pain and was diagnosed with severe life-threatening complications secondary to superior mesenteric artery thrombosis. The main objective of this case to emphasize treating physicians and surgeons about the high clinical suspicion of acute mesenteric ischemia in COVID 19 patients with acute abdominal pain and start early treatment without delay.
Case presentation
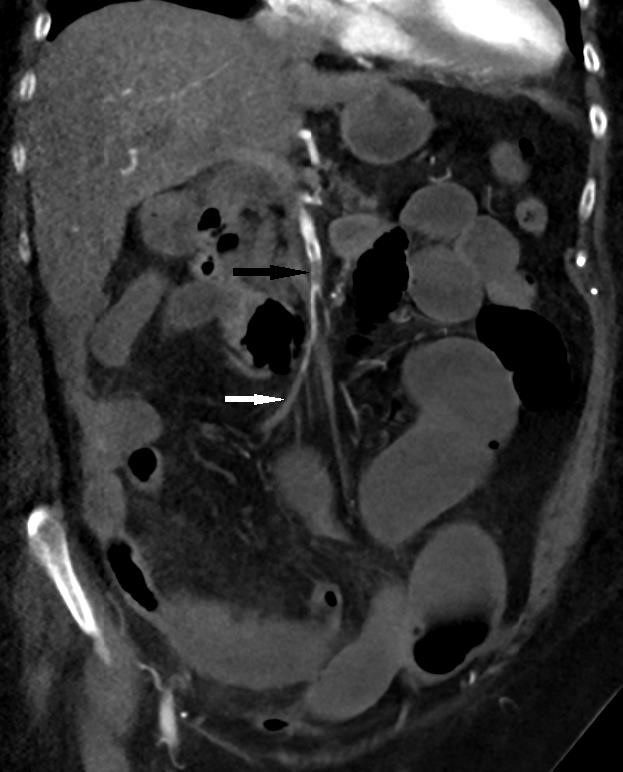
A 82-year-old female patient was admitted to the emergency department (ED) with shortness of breath and increasing severe epigastric and central abdominal pain accompanied by vomiting and diarrhea. She was tested positive for SARS -CoV-2 19 by real-time polymerase chain reaction (RT-PCR) in the community. Apart from Bronchial asthma, hypertension, hypothyroidism there were no past medical history of thromboembolic events. On physical examination, the patient was afebrile, had increased respiratory rate (24 breaths per minute) with oxygen saturation of 90% on room air. Her saturation improved to 96% with 2 liters of oxygen, heart rate was at 85 beats per minute and blood pressure was 155/85. There was an epigastric tenderness without guarding or rigidity. Laboratory examination revealed neutrophilic leukocytosis (21,500 /ul white cells with absolute neutrophils 19,700 /ul), normal platelets, elevated C-reactive protein (CRP) at 291 mg/l (standard value: <8 mg/l) and increased creatinine 299 micromol/l with a glomerular filtration rate of 33 ml/min. The liver enzymes and the lipase levels were normal. Following admission, her white cell count (WCC) and CRP continued to rise in which the results were 34,900 /ul and 388 mg/l respectively. However, her blood lactate levels were continued to be less than 2 mmol/l. Her coagulation profile on admission was INR 1.3, PT 13, APTT 43. No acute pathology was detected on Computed Tomography (CT) of the abdomen, which was performed without intravenous contrast (given the global contrast shortage at the time). She was managed with intravenous remdesivir and dexamethasone for her moderate COVID infection. Her abdominal pain was gradually settled in the initial 2 days following admission, however it started to get worsen on the third day of admission with associated worsening shortness of breath and constipation. Examination revealed evidence of severe epigastric and periumbilical tenderness with significant guarding and rigidity. Repeat multiphase CT performed with iodinated contrast demonstrated superior mesenteric artery (SMA) clot, which was nonocclusive proximally, becoming occlusive in the distal branches. Interval development of a long segment of mild small bowel dilatation with a segment of hyperdense mural thickening, mesenteric oedema and a small volume of free fluid raised concern for ischaemic enteritis. There were no features of advanced small bowel ischemia or infarction. Furthermore, CT of the chest revealed evidence of bilateral pulmonary emboli, which were appeared moderate in volume suggested significant coagulopathy. Urgent medical treatment with unfractionated heparin infusion started for anticoagulation. Given her CT abdominal finding on the time of clinical deterioration was not characteristic of bowel wall necrosis and the lactate was remained in the lower levels the surgeons decided to continue close monitoring for the next 24 hours. Unfortunately, she was rapidly deteriorated overnight, and she was taken to the theater on the following day where she underwent a 1.2 meters of small bowel resection. She also underwent a thrombo-embolectomy of the SMA thrombus during the surgery. A relook laparotomy on the following day revealed further ischemic segments and another 70 cm of her bowel was resected. Post operatively she had significant hematemesis and was performed an urgent gastroscopy. This revealed widespread gastric ulceration that unfortunately was not amenable to any endoscopic intervention. Given her significant deterioration, the decision was made to change the direction of care to a comfort base approach. She passed away peacefully following end of life care treatment.
Discussion
Since the COVID 19 pandemic, thromboembolic complications are being increasingly recognized [3]. Literature revealed several hypotheses to explain the coagulopathy in COVID 19, however the exact mechanism yet to be clarified [4]. Diffuse endothelial inflammation from the viral infection, direct invasion of bowel tissue by the virus given expression of the angiotensin converting enzyme 2 on enterocytes, virus induced cytochrome storm and increasing release of procoagulant factors such as factor V111, von Willebrand factor, fibrinogen are some of the explanations for the vasculopathy and coagulopathy in patient with SARAS-COV-2 [4]. Montagnana, M., et al., revealed that, autopsies of patient’s with COVID 19 demonstrate evidence of element of microthrombi and microangiopathic pathology, supporting virus induced in situ thrombosis rather than embolic event [11]. Acute mesenteric Ischemia (AMI) is a pathological condition characterized by a sudden decline in blood flow through the mesenteric vessels. Untreated AMI mesenteric ischemia resulting in a small bowel wall necrosis, with a significant mortality rate [5]. Broadly acute mesenteric ischemia (AMI) classified either arterial and venous ischaemia. AMI as arterial disease further classified as occlusive and non-occlusive mesenteric ischemia (NOMI). Under the occlusive mesenteric ischemia, it further classified as acute mesenteric artery thrombosis (AMAT) and acute mesenteric artery embolism (AMAE) [6]. Most of patients with AMAT have atherosclerotic disease at other sites (eg, coronary artery disease [CAD], cerebrovascular accident or stroke, or peripheral arterial disease [PAD] [4]. Absence of atheroma in the CT angiogram of abdomen as well as absence of significant past history of systemic atherosclerotic disorder suggest the clots are likely to be either due embolic event or virus induced in situ thrombosis. Studies revealed Computed tomography (CT) abdomen is the first line imaging modality for the diagnosis of acute mesenteric ischemia [7]. Lack of bowel wall enhancement, dilation and in more advanced cases pneumatosis intestinalis and portal venous gas are the characteristic CT findings closely related to intestinal necrosis [8]. CT imaging of our patent showed both occlusive and non-occlusive thromboembolic disease in the SMA. Furthermore, there was evidence of mild small bowel dilatation without other characteristic findings of advanced ischaemic enteritis, such as lack of bowel wall enhancement, or pneumatosis intestinalis. However, the histology of the small bowel resection revealed evidence of small bowel infarction with necrosis extending to the resection markings. The nonspecific early imaging findings and the unenhanced nature of initial CT were felt to have delayed the diagnosis and surgical treatment in this patient.
Regarding biochemical markers in bowel ischemia, there are no specific diagnostic test has been identified to date [10]. Increased C reactive protein (CRP), serial lactate levels, and acid-base status can guide the evaluation of patients with acute mesenteric ischaemia (AMI). However, individuals suffering from concurrent COVID-19 and AMI, elevated D-dimer, CRP, and lactate levels may be detected regardless. Hence, these results are non-specific as they may also occur in severe COVID-19 patients not suffering from AMI. Montagnana, M., et al., revealed that the raised serum lactate is considered as one of the most clinically interesting oxidative stress related biomarker [11]. Interestingly, in our patient there were no significant rise of lactate levels noted despite extensive bowel necrosis. This creates an additional obstacle to the prompt diagnosis and often delays treatment. D-dimer primarily being useful as an exclusionary test given that normal D-dimer levels are likely to exclude the potential acute thromboembolic occlusion of the SMA in a low-risk setting [10]. Furthermore, in patients with Covid 19 with coagulopathy D-dimer can predict severe and fatal cases with moderate accuracy with high sensitivity but relatively low specificity [12]. Unfortunately, our patient did not have a D-dimer level which is a limiting factor for early prediction of systemic hypercoagulable state. The intestine tolerance to ischemia becomes critical after 3–6 hours, therefore, early diagnosis for immediate therapy is essential to reach a successful outcome [9]. Studies suggest that if is there is no clinical improvement of abdominal pain in patients with acute mesenteric ischemia over a 12 to 24 h period despite intensive medical treatment, sooner the surgery is performed, the better long term results [10].
Conclusion
Acute mesenteric ischemia (AMI) is a severe, life-threatening condition that is challenging to diagnose, particularly in patients suffering from COVID-19, due to high risk of hypercoagulable state. This case is important because it once again emphasizes that in patients with COVID 19, presentation of such a detrimental, potentially fatal condition is often times vague and not necessarily textbook-like and could be easily missed due to ongoing reliance upon serum lactate level as well on CT abdomen imaging. Normal plasma lactate should not lower physician’s suspicion of the AMI in the right clinical setting in patients with known risk factors. In the context of COVID 19 coagulopathy, the clots can be disseminated in place at the same time and the imaging may be lagging behind the clinical and physical findings. Hence clinicians should combine their clinical impression along with appropriate imaging studies to obtain the best possible support for their suspected diagnosis of acute mesenteric ischemia in patients with COVID 19.
Declarations
Acknowledgment: The author acknowledges the Department of Radiology, Royal Hobart Hospital for their assistance and information regarding the radiological interpretation of this case.
Fundings: None.
Conflicts of interest (COI): None.
References
- Sukegawa M, et al., Acute superior mesenteric artery occlusion associated with COVID-19 pneumonia: a case report. Surgical Case Reports. 2022; 8: 6.
- Mahajan P, et al., COVID-19-Associated Systemic Thromboembolism: A Case Report and Review of the Literature. Cardiorenal Medicine. 2020; 10: 462-469.
- Ojha V, et al., Mesenteric ischemia in patients with COVID-19: an updated systematic review of abdominal CT findings in 75 patients. Abdom Radiol (NY). 2022; 47: 1565-1602.
- Singh B, P Kaur. COVID-19 and acute mesenteric ischemia: A review of literature. Hematol Transfus Cell Ther. 2021; 43: 112- 116.
- Lawson RM. Mesenteric Ischemia. Crit Care Nurs Clin North Am. 2018; 30: 29-39.
- Acosta S. Mesenteric ischemia. Curr Opin Crit Care. 2015; 21: 171-8.
- Demir IE, GO Ceyhan, H Friess. Beyond Lactate: Is There a Role for Serum Lactate Measurement in Diagnosing Acute Mesenteric Ischemia? Digestive Surgery. 2012; 29: 226-235.
- Taylor J, B Mandzhieva, R Shobar. Diagnosis of Acute Mesenteric Ischemia in a Patient with End-Stage Renal Disease with Normal Serum Lactate. Cureus. 2020; 12: e6708.
- Pérez-García C, et al., Non-occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery. Br J Radiol. 2018; 91: 20170492.
- Chen C, et al., Acute Mesenteric Ischemia in Patients with COVID-19: Review of the literature. J Natl Med Assoc. 2022; 114: 47-55.
- Montagnana M, E Danese, G Lippi. Biochemical markers of acute intestinal ischemia: possibilities and limitations. Ann Transl Med. 2018; 6: 341.
- Zhan H, et al., Diagnostic Value of D-Dimer in COVID-19: A MetaAnalysis and Meta-Regression. Clinical and Applied Thrombosis/ Hemostasis. 2021; 27: 10760296211010976.