Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Prognostic value of intraoperative peritoneal lavage cytology in patients with gastric cancer
Yun-tao MA1; Jia-yu XU1; Wei-peng ZHAN1; Xian-bin HUANG1; Zu-xi LI2; Chuan-wei JIN2; Jing YANG1*
1Department of the First General Surgery, Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province, Gansu Provincial Hospital, Lanzhou730000, Gansu, China.
2The First Clinical Medical College of Gansu University of Chinese Medicine, Lanzhou730000, Gansu, China.
*Corresponding Author : Jing YANG
Department of the First General Surgery, Key
Laboratory of Molecular Diagnostics and Precision
Medicine for Surgical Oncology in Gansu Province,
Gansu Provincial Hospital, Lanzhou730000, Gansu,
China.
Email: 21634604@qq.com
Received : Sep 03, 2022
Accepted : Oct 03, 2022
Published : Oct 11, 2022
Archived : www.jjgastro.com
Copyright : © YANG J (2022).
Abstract
Objective: Peritoneal metastasis of gastric cancer is common and the prognosis is generally poor. This retrospective study investigated whether peritoneal lavage cytology (CY) results may be predictive of peritoneal metastasis, and the potential risk factors for tumor outcomes.
Methods: Seventy-four patients with primary gastric cancer (cT ≥ 2) between February 2015 and November 2018 were included. We collected ascites or peritoneal lavage fluid immediately before laparoscopic exploration, and the standard cytology examination was performed. The associations between CY status and various clinicopathological features were analyzed to identify potential prognostic factors for CY1 (i.e., positive peritoneal cytology) patients.
Results: Among the 74 patients, 51 (68.9%) were CY1. Peritoneal metastasis of gastric cancer and tumor differentiation was associated with CY1. On multivariate analysis, we found that non-resection (P = 0.034) and non-HIPEC combined with systemic chemotherapy (P = 0.020) were the independent factors associated with poor survival of CY1 patients.
Conclusions: Peritoneal metastasis of gastric cancer was correlated with CY1. HIPEC combined with systemic chemotherapy and radical gastrectomy were related to overall survival of CY1 gastric cancer patients. Radical gastrectomy combined with chemotherapy may improve the prognosis of CY1 patients, which can be used as a treatment strategy for CY1 patients.
Keywords: Cytology of peritoneal lavage; Gastric cancer; Prognosis; Gastrectomy; Chemotherapy; Peritoneal metastasis.
Core tip: Published information indicates that the most common type of recurrence is peritoneal dissemination in advanced gastric cancer; however, a consensus on treatment in this area is lack. This study showed that HIPEC combined with systemic chemotherapy and radical gastrectomy were related to overall survival of CY1 gastric cancer patients. Radical gastrectomy combined with chemotherapy may improve the prognosis of CY1 patients, which can be used as a treatment strategy for CY1 patients.
Citation: Yun-tao M, Jia-yu X, Wei-peng Z, Xian-bin H, Yang J, et al. Prognostic value of intraoperative peritoneal lavage cytology in patients with gastric cancer. Japanese J Gastroenterol Res. 2022; 2(13): 1110.
Introduction
In advanced gastric cancer, the most common type of recurrence is peritoneal dissemination [1,2]. The disease outcome of patients with peritoneal metastases is generally poor, the median survival time is only a few months [3,4]. For detecting peritoneal dissemination without macroscopically observable metastatic tumors, Peritoneal Lavage (PL) cytology is a valuable tool [5]. Positivity in peritoneal lavage cytology (i.e., CY1 status) shifts the stage of the disease to M1, based on the suggestion of the Japanese Classification of Gastric Carcinoma (JCGC) [6] and the Seventh Edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual [7]. Therefore, accurate preoperative staging, especially accurate detection the free cancer cells intraperitoneally, is particularly important for prognosis and proper selection of therapy, such as Hyperthermic Intraperitoneal Chemotherapy (HIPEC), which is probably the most popular perioperative chemotherapy [8,9].
At present, the indications for intraoperative PL cytology examination in gastric cancer are controversial. For example, the guidelines of National Comprehensive Cancer Network (NCCN) recommend that, if the T3 and/or N+ patients are considered for surgical resection without preoperative therapy, intraoperative PL cytology is recommended. On the other hand, the guidelines of European Society for Medical Oncology recommend intraoperative PL cytology for patients with IB-III gastric cancer [10]. In China, early diagnosis of gastric cancer is rare, often without intraoperative PL cytology. Therefore, the currently available evidence on intraoperative PL cytology is still limited, which need more evidence-based studies to explore the value of intraoperative PL cytology [11,12].
In this study, the prognostic value of CY was investigated, and whether CY1 could be used as a surrogate biomarker to improve the accuracy of prognosis. Secondly, We discussed appropriate options of treatment for CY1 patients. To this end, the associations between CY status and various clinicopathological features were analyzed in gastric cancer patients.
Methods
Patients and methods
We retrospectively reviewed data collected from 74 gastric cancer patients who were underwent intraoperative PL cytology examination through the Center of General Surgery at the Gansu Provincial Hospital from February 2015 to November 2018. The Ethics Committee of the local Hospital approved the protocol of the study (No.16GSSY6-11).
The study population thus comprised 53 men and 21 women patients. The clinical stage (TNM classification) was determined by abdominal enhanced CT. Potential biomarkers of tumor, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and carcinoma antigen 125 (CA125) were determined via blood test. Also collected and analyzed were patients’ demographics, survival data, tumor characteristics (location, size, stage of tumor differentiation, status of tumor invasion, status of lymph node metastasis, TNM stage, and Lauren grade), peritoneal metastasis, and peritoneal cytology findings.
The definition for resection was: curative partial or total gastrectomy with D2 lymph node dissection, and the definition of non-resection was: biopsy or palliative surgery. Overall Survival (OS) was defined as the time from definitude diagnosis to the date of the last follow-up or the date of death. In the postoperative 3 years, all patients were regularly followed up, generally every 3 months. Follow-up measures contained laboratory testing, abdominal ultrasonography or CT, chest radiography, and gastroscopy. The duration of median follow-up was 18 months (from 1.0-44.0 mo).
Inclusion and exclusion criteria
All patients conformed to the following inclusion criteria: primary gastric adenocarcinoma confirmed by histopathological examination before surgery; preoperative abdominal enhanced Computed Tomography (CT) showing a clinical T2-T4 gastric cancer, based on the classification criteria of the AJCC/International Union Against Cancer (UICC) Tumor Node Metastasis (TNM) classification (7th edition); and willing to have an intraoperative PL cytology examination.
Patients were excluded from this study, if they have: history of previous neoadjuvant chemotherapy or radiotherapy; unfit for general anesthesia (including severe cardiopulmonary disease or multiple medical comorbidities); with observable peritoneal metastases at preoperative staging; or requiring laparotomy for gastric obstruction, bleeding, or perforation; patients with incomplete or inaccurate medical records.
Peritoneal lavage cytology
Immediately after the laparoscopy, and before resection of the primary tumor, the cytological examination was performed. To harvest the peritoneal lavage (in cases of no obvious ascites), warm physiologic saline (300 mL) was infused into and then drawn out from the following areas: subphrenic cavity, subhepatic cavity, omentum, bilateral paracolic sulci, and Douglas pouch.
After 5 minutes of gentle mixing (touching the primary tumor was avoided), the fluid was collected and the sediment (through centrifugation) was prepared into cell blocks and examined after Giemsa and Papanicolaou staining. If the patient was with ascites, an ascites sample was collected for the cytological examination, similar to the above-mentioned procedure (without saline infusion). A diagnosis consensus was reached by two experienced cytopathologists who examined all slides. If the sample is highly suspicious or positive for adenocarcinoma, a positive peritoneal cytology result is called.
Statistical analysis
To determine the sensitivity and specificity of cytological examination, the Receiver Operating Characteristic (ROC) curve and area under the ROC curve (AUC) were used, respectively. The statistical analyses were performed using the SPSS 22.0 statistical package (IBM, New York, USA). Specifically, in the univariate analyses of clinical and pathological variables for peritoneal cytology, the chi-squared test, or Fisher’s exact test, and Student’s t-test were used for statistical comparisons. Overall survival curves were drawn according to the Kaplan-Meier method and then the statistical difference determined by the log-rank test. Multivariate Cox regression was used to determine significant factors that were associated with prognosis. Only variables found significant on univariate analysis (P < 0.10) were considered in a multivariate model. For all analyses, P < 0.05 was judged as statistically significant.
Results
Patients’ clinicopathological features
Seventy-four patients with confirmed gastric adenocarcinoma were included in this study. All the patients underwent intraoperative PL cytology examination. Of the 74 patients, 51 (69.8%) were CY1 and 23 (30.2%) were negative (CY0). Fifty-one patients underwent potentially curative resection (35 of them were CY1). HIPEC combined with systemic chemotherapy was performed in 25 patients with positive cytology.
Significant associations were found between CY1 and peritoneal metastasis, and poor differentiation of the tumor (Table 1). None of the following were significantly associated with CY1: gender, age, surgery, tumor size, Lauren grade, tumor location, N classification, T classification, TNM stage, CEA, CA19-9, and CA125. Tumors with poor differentiation were significantly likely to be CY1 (P = 0.028). The peritoneal metastasis rate is significantly higher in CY1 patients than in CY0 patients (P = 0.008).
Sensitivity and specificity of intraoperative PL cytology examination
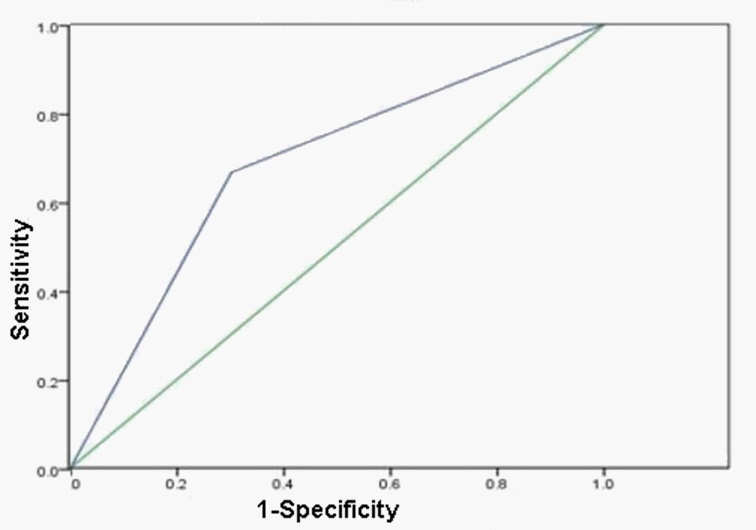
Peritoneal metastasis was confirmed by pathological examination and positron emission tomography-CT (PET-CT). Among 21 patients with peritoneal metastasis, 14 (66.7%) and 7 (33.3%) patients were CY1 and CY0, respectively. Based on the ROC curve, the sensitivity for cytology is moderate (about 66.7%) and the specificity of cytology is moderate (about 69.8%) (AUC: 0.682; Figure 1).
Table 1: The correlation between intraoperative peritoneal cytological examination results and clinicopathological features*
|
|
CY0 |
CY1 |
P |
Subjects, n |
|
23 |
51 |
|
Gender |
Male |
14(26.4) |
39(73.6) |
0.168 |
Female |
9(42.9) |
12(57.1) |
|
|
Age,y |
|
60.6±8.8 |
58.0±12.3 |
0.363 |
Surgery |
Resection |
16(37.2) |
35(62.8) |
0.936 |
No resection |
7(30.0) |
16(70.0) |
|
|
Tumor size,cmb |
|
3.9±3.2 |
3.4±2.8 |
0.722 |
Tumor locationb |
Gastric fundus & body |
13(31.7) |
28(68.3) |
0.992 |
Gastric angles & antrum |
3(30.0) |
7(70.0) |
|
|
Tumor Differentiationb |
Well |
1(50.0) |
1(50.0) |
0.028 |
Moderate |
7(30.4) |
16(69.6) |
|
|
Poor |
8(30.8) |
18(69.2) |
|
|
Lauren gradeb |
Intestinal |
9(32.1) |
19(67.9) |
0.793 |
Diffuse |
6(31.6) |
13(68.4) |
|
|
Mixed |
1(25.0) |
3(75.0) |
|
|
T classificationb |
T2 |
2(15.4) |
11(84.6) |
0.512 |
T3 |
7(41.2) |
10(58.8) |
|
|
T4 |
7(33.3) |
14(66.7) |
|
|
N classificationb |
N0 |
8(53.3) |
7(46.7) |
0.304 |
N1 |
2(25.0) |
6(75.0) |
|
|
N2 |
2(20.0) |
8(80.0) |
|
|
N3 |
4(22.2) |
14(77.8) |
|
|
Peritoneal metastasis |
P0 |
37(69.8) |
16(30.2) |
0.008 |
P1 |
7(33.3) |
14(66.7) |
|
|
TNM stageb |
IB |
1(16.7) |
5(83.3) |
0.392 |
IIA |
1(33.3) |
2(66.7) |
|
|
IIB |
5(62.5) |
3(37.5) |
|
|
IIIA |
5(38.5) |
8(61.5) |
|
|
IIIB |
3(23.1) |
10(76.9) |
|
|
IIIC |
1(12.5) |
7(87.5) |
|
|
CEA |
High |
12(42.9) |
16(57.1) |
0.121 |
Normal |
11(23.9) |
35(76.1) |
|
|
CA19-9 |
High |
10(41.7) |
14(58.3) |
0.190 |
Normal |
13(26.0) |
37(74.0) |
|
|
CA125 |
High |
11(42.3) |
15(57.7) |
0.188 |
Normal |
12(25.0) |
36(75.0) |
|
* Data is reported as n (%) unless indicated otherwise; b available after resection
Survival of CY1 patients
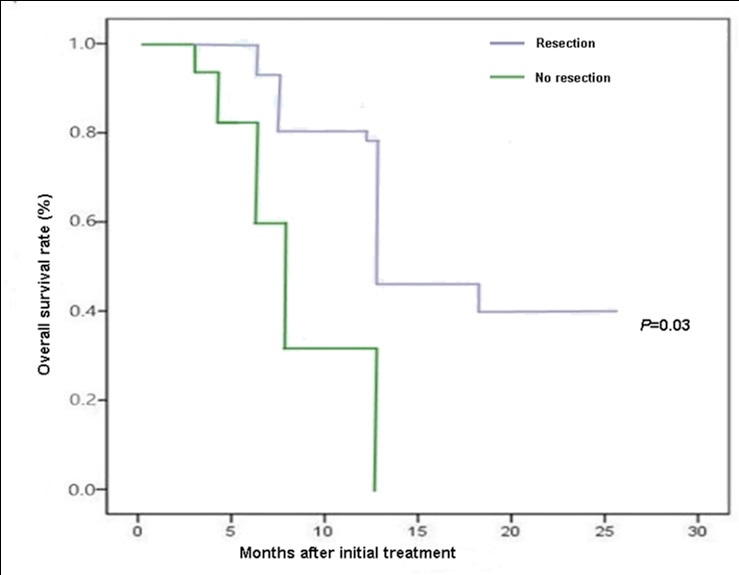
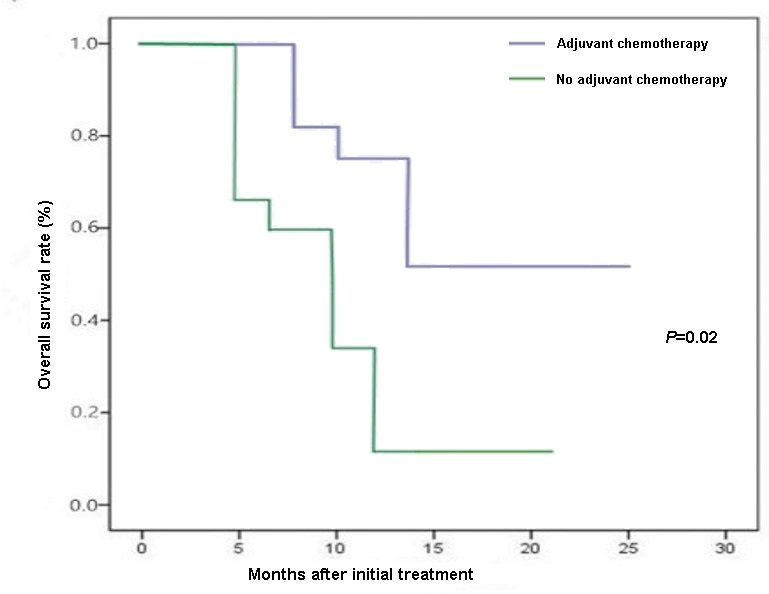
The median OS of patients with CY1 status was 11.5 months after the treatment. There is significant difference in median OS between the 35 CY1 patients who received resection (12.6 mo) and 16 CY1 patients who did not receive resection (7.6 mo; P < 0.05; Figure 2). The median OS of the 25 CY1 patients who took HIPEC combined with systemic chemotherapy (12.5 mo) was significantly better than that of the 26 CY1 patients who did not take HIPEC combined with systemic chemotherapy (10.0 mo; P < 0.05; Figure 3).
The univariate analysis suggested that the following three factors were significantly associated with prognosis of CY1 patients: resection (P = 0.04); HIPEC combined with systemic chemotherapy (P = 0.04). The multiple Cox regression analysis determined that resection (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.01-0.81; P = 0.03) and HIPEC combined with systemic chemotherapy (hazard ratio [HR], 0.07; 95% confidence interval [CI], 0.01-0.86; P = 0.02) were the independent prognostic indicators for survival of CY1 patients (Table 2).
Table 2: Univariate and multivariate analyses of prognostic factors for CY1 patients with gastric cancer.
Variables |
|
Univariate P-value |
Multivariate |
HR 95%CI P-value |
|||
Gender |
Male |
0.307 |
- - - |
Female |
|||
Age,y |
≤60 |
0.179 |
- - - |
>60 |
|||
Surgery |
Resection |
0.047a |
0.61 0.005-0.805 0.034a |
No resection |
|||
Tumor size, cmb |
≤5 |
0.284 |
- - - |
>5 |
|||
Tumor locationb |
Gastric fundus & body |
0.459 |
- - - |
Gastric angles & antrum |
|||
Tumor differentiationb |
Well |
0.515 |
- - - |
Poor |
|||
Lauren gradeb |
Intestinal |
0.413 |
- - - |
Diffuse |
|||
T classificationb |
T2-T3 |
0.449 |
- - - |
T4 |
|||
N classificationb |
N0-N2 |
0.338 |
- - - |
N3 |
|||
CEA |
High |
0.65 |
- - - |
Normal |
|||
CA19-9 |
High |
0.514 |
- - - |
Normal |
|||
CA125 |
High |
0.288 |
- - - |
Normal |
|||
HIPEC+ systemic chemotherapy |
Yes |
0.044a |
0.073 0.008-0.865 0.020a |
No |
a Significantly different; b available after resection; CI: confidence interval; HR: hazard ratio.
Discussion
The current treatment strategy for gastric cancer is comprehensive, guided by accurate staging and molecular diagnostic report. CY1 is often used as a criteria for staging, since CY1 is commonly associated with locally advanced tumors, especially those with serosal invasion [13]. However, CY1 can also be found in earlier clinical stages of gastric cancer [14,15]. Therefore, the value of CY1 as a surrogate biomarker for gastric cancer staging remains controversial.
In this study, The sensitivity and specificity of positive peritoneal cytology in predicting peritoneal metastasis were 66.7% and 69.8%. This suggests that CY1 is not a sensitive predictor of peritoneal metastasis. However, the results have indicated that the peritoneal metastasis and tumor differentiation correlated with CY1. Free cancer cells were positive in 14 (66.7%) of 21 patients with peritoneal metastasis. These results demonstrated that patients with gastric cancer carried the risk of peritoneal metastasis due to its positive cytology. It is worthwhile to mention that the reported CY1 rates vary significantly in patients with gastric cancer (from 11 to 51%) [12,16]. This may partially be attributed to differences in methodology among studies [17,18], since the technique of peritoneal cytology has not been standardized. Alternatively, the high CY1 rate in the present study (overall CY1 rate = 68.9%) may reflect sampling bias, but it may also attribute to the cell block technique in this study.
Regarding the optimal treatment for patients with CY1, there is also no consensus. The current NCCN guidelines consider CY1 to be a criterion of unrespectability for cure [19], while the JCGC recommends that peritoneal cytology should be used for staging and prognostic purposes only [20]. The main treatments of gastric cancer include endoscopic therapy, surgery, chemotherapy, radiotherapy, immunotherapy and so on [21,22]. We found that the gastrectomy and HIPEC combined with systemic chemotherapy were independent prognostic factors in the present CY1 patient cohort. Lee SD et al. [23] and Suzuki et al. [24] reported a similar finding. Our study showed a survival benefit of standard radical gastrectomy combined chemotherapy for patients with CY1. However, the therapeutic strategy for CY1 patients could not be easily decided, likely due to selection bias of this study. Randomized controlled studies of gastrectomy combined postoperative chemotherapy in CY1 patients with gastric cancer are rare. Based on the results of CCOG301, adjuvant chemotherapy for CY1 patients with gastric cancer could be considered after radical surgery [25]. It has been reported that radical surgery combined postoperative S-1 monotherapy can make the median OS of CY1 patients reach 22.3 months [26]. At our center, the specific strategy for gastric cancer patients with CY1 is determined curative surgery and postoperative HIPEC combined with systemic chemotherapy. Several diverse therapeutic strategies that primarily target peritoneal dissemination have been explored. Fujimura et al. [27] reported that postoperative chemotherapy and HIPEC could increase the 3-year overall survival rate of patients with CY1, and more importantly, a meta-analysis confirmed this finding [12]. We tend to believe that peritoneal cytology could be a useful marker in identifying patients who are probably benefit from these therapeutic options. HIPEC and systemic chemotherapy should be tried for those patients with a poor prognosis.
Regarding the prognostic value of CY1, many reports have suggested a close association between peritoneal metastasis and CY1, therefore indicating that CY1 may predict peritoneal metastasis in gastric cancer patients [11,23,28,29]. Nevertheless, some studies did suggest that CY1 alone made no contribution to the available prognostic information [12]. Therefore, the exact prognostic value of CY1 remains inconclusive.
The present retrospective study is limited, in that it was performed at a single center. Secondly, the number of gastric cancer patients is relatively low, which may have led to the lack of a statistically significant association between CY and OS. Finally, the possibility of selection bias cannot be excluded. Therefore, further multi-centered studies with a larger series of patients are warranted.
Conclusion
Overall, in this study, a strong correlation was found between CY1 and peritoneal metastasis of gastric cancer. To improve prognosis of CY1 patients, radical gastrectomy and HIPEC combined with systemic chemotherapy should be considered. The intraoperative PL cytology examination is probably necessary to determine treatment strategy in patients with gastric cancer.
Declarations
Ethics approval and consent to participate: The Ethics Committee of the local Hospital approved the protocol of the study (No.16GSSY6-11).
Consent for publication: All data published here are under the consent for publication.
Availability of data and material: The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Conflict of interest: All authors do not have any conflict of interests to declare.
Acknowledgements: None.
Authors’ contributions: JY and YTM designed/performed most of the study, data analysis and wrote the manuscript; JYX, ZXL and CWJ provided pathological assistance; XBH and WPZ contributed to interpretation of the data. All authors approved the final version for publication.
Funding: This work was supported by Gansu Natural Science Foundation (No.20JR10RA403 and No. 21JR1RA017).
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021; 71: 209-249.
- Yamaguchi T, Takashima A, Nagashima K, Terashima M, Aizawa M, et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. GASTRIC CANCER. 2021; 24: 701-709.
- Kang W, Zhong Y, Ma F, Xue L, Xiong J, et al. Survival outcomes and prognostic indicators for gastric cancer patients with positive peritoneal wash cytology but no peritoneal metastasis after radical gastrectomy. WORLD J GASTRO ONCOL. 2021; 13: 24-36.
- Li BL, Miao RL, Li ZY. Progress in gastric cancer with positive peritoneal cytology. Zhonghua Wei Chang Wai Ke Za Zhi. 2021; 24: 458-462.
- Endo S, Nishikawa K, Ikenaga M, Fujitani K, Kawada J, et al. Prognostic factors for cytology-positive gastric cancer: a multicenter retrospective analysis. INT J CLIN ONCOL 2021 2021-01-01; 26(5): 858-866.
- Japanese GCA. Japanese classification of gastric carcinoma: 3rd English edition. GASTRIC CANCER. 2011; 14: 101-112.
- Washington K. 7th edition of the AJCC cancer staging manual: stomach. ANN SURG ONCOL. 2010; 17: 3077-3079.
- Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, et al. Cytoreductive Surgery with or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J CLIN ONCOL. 2019; 37: 2028-2040.
- Chia D, So J. Recent Advances in Intra-peritoneal Chemotherapy for Gastric Cancer. J GASTRIC CANCER. 2020; 20: 115-126.
- M Chen LT, Nakajima TE, Shitara K, Kawakami H, Tsushima T, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMOESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. ANN ONCOL. 2019; 30: 19-33.
- Yamaguchi T, Takashima A, Nagashima K, Terashima M, Aizawa M, et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. GASTRIC CANCER. 2021; 24: 701-709.
- Jamel S, Markar SR, Malietzis G, Acharya A, Athanasiou T, et al. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. GASTRIC CANCER. 2018; 21: 10-18.
- Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. ANN SURG. 2018; 267: 105-113.
- Dong D, Tang L, Li ZY, Fang MJ, Gao JB, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. ANN ONCOL. 2019; 30: 431-438.
- Pak LM, Coit DG, Eaton AA, Allen PJ, D’Angelica MI, et al. Percutaneous Peritoneal Lavage for the Rapid Staging of Gastric and Pancreatic Cancer. ANN SURG ONCOL. 2017; 24: 1174-1179.
- Yasufuku I, Nunobe S, Ida S, Kumagai K, Ohashi M, et al. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. GASTRIC CANCER. 2020; 23: 319-327.
- Qu L, He L, Jia Z, Wang Q. Prognostic value of CEA/CA72-4 immunohistochemistry in combination with cytology for detecting tumor cells in peritoneal lavage in gastric cancer. J CANCER. 2020; 11: 6319-6325.
- Kołomańska MM, Głuszek S. Free cancer cells in gastric cancer - methods of detection, clinical and prognostic importance (meta-analysis). Contemp Oncol (Pozn). 2020; 24: 67-74.
- Ajani JA, Barthel JS, Bekaii-Saab T, Bentrem DJ, D’Amico TA, et al. Gastric cancer. J Natl Compr Canc Netw. 2010; 8: 378-409.
- Japanese gastric cancer treatment guidelines 2014 (ver. 4). GASTRIC CANCER. 2017; 20: 1-19.
- Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? ANN SURG ONCOL. 2008; 15: 2684-2691.
- Kawazoe A, Shitara K, Boku N, Yoshikawa T, Terashima M. Current status of immunotherapy for advanced gastric cancer. JPN J CLIN ONCOL. 2021; 51: 20-27.
- Lee SD, Ryu KW, Eom BW, Lee JH, Kook MC, et al. Prognostic significance of peritoneal washing cytology in patients with gastric cancer. Br J Surg. 2012; 99: 397-403.
- Suzuki O, Fukuchi M, Mochiki E, Ishiguro T, Sobajima J, et al. Prognostic role of gastrectomy in patients with gastric cancer with positive peritoneal cytology. INT SURG. 2014; 99: 830-834.
- Kano K, Aoyama T, Maezawa Y, Nakajima T, Ikeda K, et al. The survival and prognosticators of peritoneal cytology-positive gastric cancer patients who received upfront gastrectomy and subsequent S-1 chemotherapy. Int J Clin Oncol. 2017; 22: 887-896.
- Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013; 119: 3354-3358.
- Fujimura T, Yonemura Y, Kawamura T, Nojima N, Satoh T, et al. Cytoreductive surgery and sandwich therapy with chemohyperthermic peritoneal perfusion and intra-aortic chemotherapy for peritoneal dissemination in gastric cancer. ONCOL REP. 1996; 3: 513-517.
- Hoskovec D, Varga J, Dytrych P, Konecna E, Matek J. Peritoneal lavage examination as a prognostic tool in cases of gastric cancer. ARCH MED SCI. 2017; 13: 612-616.
- Graziosi L, Cantarella F, Mingrone E, Gunnellini M, Cavazzoni E, et al. Preliminary results of prophylactic HIPEC in patients with locally advanced gastric cancer. ANN ITAL CHIR. 2013; 84: 551- 556.