Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
EUS guided portal vein sampling in patients with suspected pancreatic adenocarcinoma: Experience from a single centre
Vinh-An Phan1,2; Andrew Ruszkiewicz1,3; Joel Geoghegan4 ; Nam Q Nguyen1,2*
1Department of Gastroenterology and Hepatology, Royal Adelaide Hospital, North Terrace, Adelaide, South Australia, Australia.
2Discipline of Medicine, University of Adelaide, North Terrace, Adelaide, South Australia, Australia.
3Gastroenterology Research Laboratory, SA Pathology, Frome Road, Adelaide, South Australia, Australia.
4ACRF SA Cancer Genomics Facility, IMVS, Frome Road, Adelaide, South Australia, Australia.
*Corresponding Author : Nam Q Nguyen
Department of Gastroenterology and Hepatology,
Royal Adelaide Hospital, North Terrace, Adelaide,
South Australia, 5000, Australia.
Tel: +61-8-8222 5214, Fax: +61-8-8222-5885;
Email: quocnam.nguyen@sa.gov.au
Received : Jun 19, 2022
Accepted : Jul 22, 2022
Published : Jul 29, 2022
Archived : www.jjgastro.com
Copyright : © Nguyen NQ (2022).
Abstract
Background: Studies looking at EUS guided portal vein sampling have largely been limited to live porcine models with only few reports on human trials. Indications so far, have included portal pressure measurement and liquid biopsies to assist in precision medicine for cancer patients.
Aim: To assess the safety and feasibility of EUS guided portal vein sampling in patients with suspected pancreatic cancer. Methods: Patients undergoing EUS FNA for suspected pancreatic masses were recruited. Each subject subsequently underwent EUS guided portal venous sampling with a 22G FNA device. A combined trans-gastric and trans-hepatic approach was used. Patients were assessed for immediate and early complications. Blood specimens were analysed for circulating cell-free DNA (cfDNA). Results: 16 patients were recruited. The final diagnoses included PDAC (n=11), metastatic squamous cell carcinoma (n=1), Acinar Cell Carcinoma (n=1) and pancreatitis (n=3). Technical success rate was 100% with no complications secondary to the portal venous sampling. Detectable levels of cfDNA were found in all analysed samples. Conclusion: This study demonstrates the safety and high success rate of a trans-gastric/trans-hepatic EUS guided portal vein sampling in patients with suspected pancreatic cancer. We recommend this approach to reduce bleeding risk.Keywords: Pancreatic cancer; Endoscopic ultrasound; Liquid biopsy.
Citation: Phan VA, Ruszkiewicz A, Geoghegan J, Nguyen NQ. EUS guided portal vein sampling in patients with suspected pancreatic adenocarcinoma: Experience from a single centre. Japanese J Gastroenterol Res. 2022; 2(11): 1099.
Introduction
When initially introduced, portal venous sampling was used as a diagnostic tool in the investigation of neuroendocrine tumours. Done as a radiological guided procedure, it has been shown to be safe and accurate when used to localise gastrinomas and insulinomas in conjunction with gastrin and insulin assays respectively [1-3]. The confluence of the superior mesenteric and splenic veins forms the portal vein. It conducts blood to the liver, which then acts as a filter [4]. Therefore, sampling this blood before it is filtered (as opposed to peripheral blood), is a source of potentially higher yield for liquid biopsy in the investigation of pancreatic cancer.
Endoscopic Ultrasound guided fine needle aspiration (EUS FNA) has become the preferred method for sampling lesions within and adjacent to the gastrointestinal tract. It is safe with sensitivity, specificity and diagnostic accuracy consistently exceeding 85% [5,6]. Given that the portal vein is easily visualised during EUS, it provides a logical avenue for portal venous sampling.
In this study, we report the safety and technical success rate of EUS guided portal venous sampling in patients with suspected Pancreatic Ductal Adenocarcinoma (PDAC). The samples were further analysed to quantify cell free DNA as a potential method of biological cancer staging.
Methods
We recruited patients referred to our unit for EUS guided FNA for suspected PDAC (regardless of stage). This study was approved by the Research Ethics Department at the Royal Adelaide Hospital.
Inclusion criteria
(i) Metastatic pancreatic cancer: imaging evidence of a pancreatic mass with overt discrete lesion(s) in the liver or distant organs.
(ii) Locally advanced pancreatic cancer: imaging evidence of a pancreatic mass that involved the adjacent organs or vasculatures (Superior Mesenteric Vein, Superior Mesenteric Artery, Portal Vein, or hepatic artery), without overt evidence of hepatic or distant metastasis.
(iii) Resectable Pancreatic Cancer: imaging evidence of an isolated mass in the pancreas without involvement of adjacent organs, vasculatures, or distant metastasis.
Exclusion criteria
(i) Coagulopathy (INR > 1.4) or Platelet count less than 50 x 109 /L.
(ii) Complete portal vein thrombosis, especially those extending into the hilum and liver.
(iii) Portal Hypertension (clinically, endoscopic features or EUS features).
Peri-procedure management
All patients gave informed consent and management of anti-coagulant and anti-platelet medications were organised prior to the procedure. Patients were given prophylactic intravenous antibiotics within 30 minutes of the procedure (1 gm Amoxycillin, 500 mg Metronidazole and 6 mg/kg dose of Gentamicin; regime was adjusted according to allergies and renal function).
A 10 ml peripheral blood sample was taken before EUS FNA of the pancreatic lesion. Two ml of this sample was sent to check haemoglobin levels and the remaining reserved for circulating cell free DNA analysis.
The procedure
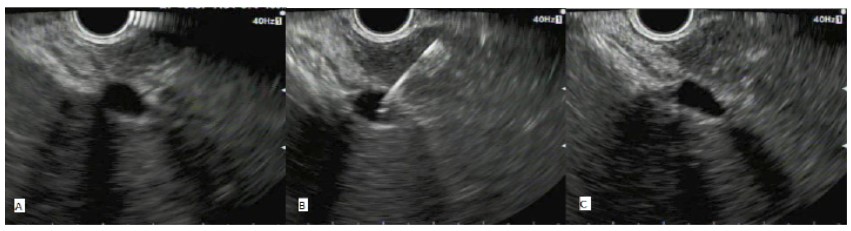
The procedure was performed by a highly experienced endosonographer (over 5000 procedures performed). EUS guided portal venous sampling was done following standard EUS FNA of the mass lesion with a linear-array echoendoscope (Figure 1):
(i) With the tip of the echoendoscope located along the lesser curvature of the stomach (mid-body) the main portal vein is located.
(ii) This is followed to the right and left portal vein bifurcation and the intra-hepatic left branch is targeted for fine needle aspiration.
(iii) Using the distance function, at least 1-1.5 cm of hepatic parenchyma is measured to be traversed for FNA of portal venous blood (i.e. there is 1-1.5 cm of hepatic tissue between the scope tip and the vein puncture site).
(iv) A 22G FNA needle is used to puncture the left main branch of the portal vein and the stylet withdrawn.
(v) 8 ml of venous blood is aspirated into a 25 ml syringe (we allowed a maximum time of 150 seconds to prevent coagulation of blood in the EUS needle channel).
(vi) After the needle is withdrawn the site is reassessed under EUS to check for immediate bleeding.
(vii) The remaining blood within the FNA needle channel is flushed with air into the blood collection tube.
Post-procedure
Patients were monitored for a minimum of 90 minutes in our recovery unit for complications (including abdominal pain, fever, perforation, and suspected bleeding). Haemoglobin levels were checked (at day 1 or 2 post-procedure) and patients were called after 3 days to check for concerning symptoms.
Cell free DNA extraction
Blood samples (peripheral and portal venous) were centrifuged to remove cells and cell components. Qiagen circulating nucleic acid extraction kits were used to isolate circulating cell free DNA. Yields of circulating cfDNA were quantified (ng/ml) to compare the 2 different sources of blood.
Statistical analysis
Statistical analysis was carried out using SPSS version 22.0.0 for Windows. Quantitative variables were tested by t-test with P<0.05 considered statistically significant. Statistics were reported as mean ± standard deviation.
Results
Sixteen patients (M:F=1:1) were recruited for this trial and all had pancreatic masses on pre-procedure imaging, suspicious for PDAC. The median age was 72 years (range 51-87). The final diagnoses included PDAC (n=11), metastatic squamous cell carcinoma (n=1), Acinar Cell Carcinoma (n=1) and pancreatitis (n=3) (Table 1).
All patients underwent successful EUS guided portal venous sampling with a mean 8.3 ± 0.44 mL of blood obtained in each case. We used the trans-gastric/trans-hepatic approach in all cases. Aspiration of blood took at least 120 seconds in each case (maximum 150 seconds).
There were no cases of immediate post-procedure bleeding or gastrointestinal perforation. Three patients (18.8%) had preexisting abdominal pain that was not worse post-procedure (1 week follow up). Two patients (12.5%) with PDAC reported new post-procedure epigastric pain within 1 hour of completion. However, both also underwent ERCP and stenting for biliary strictures immediately following EUS FNA/portal venous sampling. In both cases, pain responded well to single dose Acetaminophen (1 gm IV) with complete resolution within 24 hours. Mean pre- and post-procedure haemoglobin levels were 128.4 ± 14.6 g/L and 126.7 ± 17 g/L (P=0.41).
One patient (51-year-old female with PDAC) was admitted post-procedure with complete heart block. This was discovered on cardiac monitoring during the EUS FNA but prior to portal vein sampling. This is not thought to be related to our intervention.
Seven (PDAC=4, Pancreatitis=1, ACC=1, SCC=1) of patients had their samples processed in order to isolate and quantify circulating cfDNA (Table 2). The mean quantity (ng/ml) of cfDNA isolated in the peripheral versus portal venous blood was 79.7 ± 155.2 vs 25.7 ± 43.4 respectively (P=0.39). When comparing PDAC patients only, the mean quantity (ng/ml) of peripheral versus portal venous blood was 34.5 ± 57.7 vs 32.0 ± 52.7 respectively (P=0.95). The highest portal venous cfDNA yield was seen in a patient with PDAC stage T3N1M0 (121 ng/ml). The highest peripheral venous yield was seen in a patient with metastatic SCC (420 ng/ml).
(A): With the tip of the echoendoscope located along the lesser curvature of the stomach (mid-body) the main portal vein is located. This is followed to the right and left portal vein bifurcation and the intra-hepatic left branch is targeted for fine needle aspiration.
(B): Using the distance function, we ensure a 1-1.5cm segment of hepatic tissue is between the needle insertion site and the vein. A 22G FNA needle is used to puncture the left main branch of the portal vein and the stylet withdrawn for sampling.
(C): After the needle is withdrawn the site is reassessed under EUS to check for immediate bleeding.
Table 1: Patient Summary and Outcome.
| Patient Number | Gender | Age (years) | Diagnosis | Portal Venous Sample Volume (ml) | Pre-procedure Hb (g/L) | Post-procedure Hb (g/L) | Adverse Events |
|---|---|---|---|---|---|---|---|
| 1 | F | 87 | PDAC | 8 | 105 | 111 | Pre-existing epigastric pain-not worsened |
| 2 | F | 74 | PDAC | 8 | 144 | 150 | Nil |
| 3 | M | 66 | PDAC | 8.5 | 110 | 110 | Nil |
| 4 | M | 68 | PDAC | 8.5 | 154 | 140 | Nil |
| 5 | M | 54 | PDAC | 8 | 123 | 109 | Pre-existing epigastric pain-not worsened |
| 6 | F | 75 | Pancreatitis | 8 | 121 | 115 | Nil |
| 7 | M | 68 | Pancreatitis | 8 | 156 | 161 | Nil |
| 8 | F | 57 | PDAC | 9 | 131 | 131 | Nil |
| 9 | M | 75 | Pancreatitis | 8 | 138 | 150 | Nil |
| 10 | M | 73 | PDAC | 8 | 121 | 121 | Epigastric pain* |
| 11 | F | 51 | PDAC | 8 | 121 | 126 | Complete heart block mid-procedure requiring admission |
| 12 | F | 76 | PDAC | 8 | 129 | 128 | Nil |
| 13 | F | 83 | PDAC | 8 | 136 | 129 | Epigastric Pain* |
| 14 | F | 58 | ACC | 9 | 128 | 131 | Nil |
| 15 | M | 79 | Metastatic SCC | 9 | 111 | 107 | Nil |
| 16 | M | 71 | PDAC | 9 | 126 | 114 | Pre-existing epigastric pain -not worsened |
*Underwent ERCP for stenting of biliary stricture immediately after EUS
PDAC: Pancreatic Ductal Adenocarcinoma; ACC: Acinar Cell Carcinoma; SCC: Squamous Cell Carcinoma
Table 2: Results from circulating cfDNA isolation
| Patient number | Diagnosis | PDAC Stage | Portal Venous cfDNA in plasma (ng/ml) | Peripheral Venous cfDNA in plasma (ng/ml) | Ratio Portal Vein / Peripheral Vein cfDNA |
|---|---|---|---|---|---|
| 8 | PDAC | T3N1M0 | 3 | 2 | 1.5 |
| 9 | Pancreatitis | NA | 2 | 2 | 1.0 |
| 11 | PDAC | T1N0M0 | 5 | 8 | 0.625 |
| 12 | PDAC | T3N0M0 | 9 | 7 | 1.29 |
| 13 | PDAC | T3N1M0 | 121 | 111 | 1.09 |
| 14 | ACC* | NA | 6 | 8 | 0.75 |
| 15 | SCC# | NA | 34 | 420 | 0.081 |
*No metastatic or nodal involvement
#Known Squamous Cell Carcinoma with metastatic involvement of pancreas cfDNA: cell free DNA; NA: Not Applicable; PDAC:
Pancreatic Ductal Adenocarcinoma; ACC: Acinar Cell Carcinoma; SCC: Squamous Cell Carcinoma
Discussion
The ability of an echoendoscope to come within close proximity of intra-abdominal lesions and vessels allows high resolution imaging while avoiding the need to traverse other organs. This makes it the logical device to use for accessing the portal vein. In this single centre study, we obtained 8ml portal venous samples in 16 patients with 100% success rate and without immediate or short-term complications. Trans-gastric sonographic views of the portal vein were excellent in all cases.
We have employed a combined trans-gastric and trans-hepatic approach using a 22G FNA needle. Our theory being that the liver tissue provides a cushion of pressure on the portal vein defect after withdrawal of the needle. Immediate EUS assessment of the puncture site was done in all cases, with no evidence of bleeding. We have yet to use a trans-duodenal technique. In our literature search looking at EUS guided portal venous access, Huang et al (n=28) and Rustagi et al (n=12) both used a trans-duodenal or trans-gastric/hepatic approach depending on access. Neither approach resulted in complications [7, 8]. However, the safety of the trans-duodenal approach has not been reported as extensively in humans. Also, neither Huang et al or Rustagi et al stated what proportion of patients underwent the trans-duodenal versus the trans-gastric route.
We chose to use the 22G FNA needle, given the thinner calibre results in a smaller portal vessel defect. The drawback was blood aspiration took at least 2 minutes in all cases and there was concern that clots would form within the syringe and needle channel. Catenacci et al used a trans-hepatic approach with a 19G FNA needle in 18 patients to take 7.5ml portal venous samples with 100% success rate and no complications [9]. Four studies involving 25 live porcine models also used a 19G needle with no technical failures and without complications [10-13]. This suggests using a larger 19- or 20-gauge needle is safe, may reduce the time needed to aspirate the blood sample and allow a larger sample to be taken.
We acknowledge several steps in our methods, which need review. Our exclusion criteria included those with portal hypertension and/or portal vein thrombosis. The main concern being an increased risk of bleeding caused by the higher pressure. This precaution was probably unnecessary given multiple studies involving patients with portal hypertension or portal vein thrombosis have not had post-procedure bleeding [7,8,14]. Also, all of our patients received prophylactic IV antibiotics prior to the procedure with no infectious complications occurring. It should be noted however, that this complication has yet to be reported in the literature with or without the administration of antibiotics.
To date, the studies looking at portal venous sampling have been in the pilot phase. Indications have varied between portal pressure measurement to liquid biopsy for cancer staging [7,8]. Liquid biopsy has emerged as an alternative to traditional solid tissue biopsy for both diagnostic and prognostic purposes in the work up of cancer patients [15]. Circulating cfDNA is released from cells following apoptosis [16]. In our pilot study, we assessed the safety and feasibility of using this technique to detect cell-free DNA in those with suspected PDAC. In patients with pancreatic cancer, Bettegowda et al were able to detect tumour specific cfDNA from peripheral blood samples. A higher proportion of these patients with detectable tumour specific cfDNA were those with metastatic disease, indicating that this may be a potential biomarker [17]. We have been able to successfully obtain cfDNA from EUS guided portal venous samples. There was no significant difference in terms of the quantity of cfDNA in peripheral versus portal samples. However, this was not tumour specific DNA and reflects the additional cfDNA from apoptosis of normal tissue cells.
Bettegowda et al had a cell free tumour DNA detection rate of 48% in peripheral venous blood from their pancreatic cancer patients [17]. The next step in our research will be quantifying the small amount of cfDNA originating specifically from tumour cells (as opposed to normal cells) in the portal blood. This may have a higher yield compared to peripheral samples and hence become a more sensitive method of biologically staging pancreatic cancer
Conclusions
In conclusion, this study again demonstrates the safety and high success rate of a trans-gastric/trans-hepatic EUS guided portal vein sampling in humans. We recommend this approach to reduce bleeding risk. Further studies should focus on indications for this procedure and in particular, how it can contribute to diagnosis, staging and treatment guidance in pancreaticobiliary cancers.
Authors contributions
Vinh-An Phan: manuscript preparation, literature search, clinical studies, data acquisition, data analysis, statistical analysis Andrew Ruszkiewicz: concept, design
Joel Geoghegan: concept, design, data analysis, data acquisition.
Quoc Nam Nguyen: concept, design, clinical studies, manuscript review.
References
- Miller DL, Doppman JL, Metz DC, Maton PN, Norton JA, et al. Zollinger-Ellison syndrome: technique, results, and complications of portal venous sampling. Radiology. 1992; 182: 235-41.
- Thom AK, Norton JA, Doppman JL, Miller DL, Chang R, et al. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992; 112: 1002-8.
- Doppman JL, Chang R, Fraker DL, Norton JA, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial stimulation with calcium. Ann Intern Med. 1995; 123: 269-73.
- Mourad N, Zhang J, Rath AM, Chevrel JP. The venous drainage of the pancreas. Surg Radiol Anat. 1994; 16: 37-45.
- Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75: 319-31.
- Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011; 73: 283-90.
- Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, et al. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. Gastrointest Endosc. 2017; 85: 996-1001.
- Rustagi T, Gleeson FC, Chari ST, Abu Dayyeh BK, Farnell MB, Iyer PG, et al. Remote malignant intravascular thrombi: EUS-guided FNA diagnosis and impact on cancer staging. Gastrointest Endosc. 2017; 86: 150-5.
- Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, et al. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015; 149: 1794-803 e4.
- Giday SA, Clarke JO, Buscaglia JM, Shin EJ, Ko CW, Magno P, et al. EUS-guided portal vein catheterization: a promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008; 67: 338-42.
- Buscaglia JM, Dray X, Shin EJ, Magno P, Chmura KM, Surti VC, et al. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video). Gastrointest Endosc. 2009; 69: 941-7.
- Schulman AR, Ryou M, Aihara H, Abidi W, Chiang A, Jirapinyo P, et al. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: a novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest Endosc. 2017; 85: 243-7.
- Buscaglia JM, Shin EJ, Clarke JO, Giday SA, Ko CW, Thuluvath PJ, et al. Endoscopic retrograde cholangiopancreatography, but not esophagogastroduodenoscopy or colonoscopy, significantly increases portal venous pressure: direct portal pressure measurements through endoscopic ultrasound-guided cannulation. Endoscopy. 2008; 40: 670-4.
- Kayar Y, Turkdogan KA, Baysal B, Unver N, Danalioglu A, Senturk H. EUS-guided FNA of a portal vein thrombus in hepatocellular carcinoma. Pan Afr Med J. 2015; 21: 86.
- Li BT, Stephens D, Chaft JE, Rudin CM, Jones DR, Rusch VW, et al. Liquid biopsy for ctDNA to revolutionize the care of patients with early stage lung cancers. Ann Transl Med. 2017; 5: 479.
- Marzese DM, Hirose H, Hoon DS. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev Mol Diagn. 2013; 13: 827-44.
- Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014; 6: 224ra24.