Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Laparoscopic pancreaticoduodenectomy: The initial experience at a low volume centre
Ameet Kumar*; Sumesh Kaistha
Department of GI Surgery, Command Hospital, Bangalore, India.
*Corresponding Author : Ameet Kumar
Department of GI Surgery, Command Hospital, Pune,
India.
Tel: +919013818845; Email: docam@rediffmail.com
Received : Jun 04, 2022
Accepted : Jun 30, 2022
Published : Jul 07, 2022
Archived : www.jjgastro.com
Copyright : © Kumar A (2022).
Abstract
Purpose: Pancreaticoduodenectomy (PD) is considered as one of the most complex gastrointestinal surgery. A totally laparoscopic approach to PD (LPD) has been daunting for most surgeons owing to its steep learning curve and the technically demanding reconstruction phase. Reports from low volume centres have raised concerns regarding higher morbidities and even mortality. We present our initial experience with LPD at a low volume centre
Methods: This was a retrospective review of all patients who underwent LPD with an aim to look at the short term outcomes.
Results: LPD was attempted in 13 patients and could be successfully completed in 10 patients (conversion rate of 23%); 09 of these had a soft pancreas with small duct. The median operative time was 460 (430–550) minutes; median blood loss was 480 (150–1000) ml; the overall morbidity rate was 40%; the median length of stay was 14 (7-30) days and there was a single mortality in a patient who had a grade C pancreatic fistula. The median number of lymph node resected was 18 and all patients had a R0 resection.
Conclusion: The results were comparable with the studies from high volume centres reporting their respective initial LPD case series. With a diligently planned learning path and audit, LPD is feasible and can be safely done even at a low volume center.
Keywords: Pancreaticoduodenectomy; Laparoscopic; Low volume centre; Initial experience; Short term outcomes.
Abbreviations: PD: Pancreaticoduodenectomy; PE: Pancreaticoenetric; LLR: Laparoscopic liver resection; MAS: Minimal access surgery; LPD: Totally laparoscopic pancreaticoduodenectomy; LDP: Laparoscopic distal pancreatectomy; LVC: Low volume centre; PBD: Preoperative biliary drainage; PJ: Pancreaticojejeunostomy; PG: Pancreaticojejunostomy; HJ: Hepaticojejunostomy; GJ: Gastrojejunostomy; POD: Post operative day; POPF: Post operative pancreatic fistula; ISGPS: International study group for pancreatic surgery.
Citation: Kumar A, Kaistha S. Laparoscopic pancreaticoduodenectomy: The initial experience at a low volume centre. Japanese J Gastroenterol Res. 2022; 2(10): 1094.
Introduction
Pancreaticoduodenectomy (PD) is considered as one of the most demanding gastrointestinal (GI) surgery that entails a complex resection involving dissection in an anatomically crucial area where anomalies are a rule rather than an exception. Pancreaticoenteric (PE) anastomosis, which is the Achilles’ heel of PD, is a technically demanding reconstruction adding to the complexity of PD. Although the mortality rate following PD has decreased from 38% to <5% in high-volume centers, the high morbidity, with rates ranging from 40% to 60%, remains a cause of concern [1-4].
Laparoscopic pancreatic resection along with laparoscopic liver resection (LLR) is considered the final frontier in minimal access surgery (MAS) of the GI system [5]. Some believe that a total laparoscopic PD (LPD) is more challenging than LLR and is the most advanced application of laparoscopic surgery.
Although laparoscopic distal pancreatectomy (LDP) has gained universal acceptance [6], the same is not true for LPD because of its steep learning curve and the need for challenging reconstruction that can lead to suboptimal outcomes [7-9]. Retrospective studies and meta-analyses have reported that LPD can result in shorter hospital stay, decreased blood loss, lower pain, and earlier return to work at the cost of increased operating time [10]. Two of three randomized controlled trials (RCTs; PLOT, PADULAP, and LEOPARD-2 trials) have reported the benefits of LPD, and a meta-analysis of these RCTs supported the use of LPD [10-13]. However, studies conducted in low-volume centers (LVCs) have raised concerns regarding high morbidity and even mortality [14].
Here, we describe our experience of performing LPD at an LVC by focusing on short-term outcomes and discussing the path of progression toward LPD.
Methods
We retrospectively reviewed the data of all patients who underwent LPD between July 2016 and Feb 2019. All patients were operated upon by two authors with adequate experience in hepato-bilio-pancreatic and advanced laparoscopic surgery. This study aimed to examine the short-term outcomes of patients with the objective of assessing the conversion rates, operative time, blood loss and need for transfusion, overall morbidity, pancreas-specific complications, reoperation, readmission, mortality, and oncological safety. The approval of the ethical committee was not required prior to starting LPD because it is a well-described surgery and has been used in previous studies. However, we provided a full disclosure regarding the surgical team’s status with respect to LPD to patients. The study protocol was approved by the ethical committee (IEC/ MED/2021/08).
Preoperative evaluation
All patients underwent a complete clinical evaluation, with a focus on performance status and optimization of comorbidities. Standard relevant laboratory investigations were undertaken and included serum tumor markers. A contrastenhanced computerized tomography scan with pancreatic protocol was performed in all patients. Magnetic resonance imaging was selectively performed at the discretion of the surgeon. Side viewing endoscopy was done in an attempt to establish a diagnosis and biopsy. Patients with a serum bilirubin level >20 mg/dL or in cholangitis underwent a preoperative biliary drainage (PBD).
We included patients with periampullary or pancreatic cancer with favorable vascular anatomy, as demonstrated on preoperative imaging. All patients were provided information regarding the procedure, the risks and benefits of the laparoscopic procedure, and the possibility of conversion to an open procedure. All cases were discussed in the tumor board. We excluded patients who underwent multiple abdominal surgeries previously, those with any suspicion of vascular involvement, and those who refused to consent to undergo a laparoscopic procedure.
Operative procedure
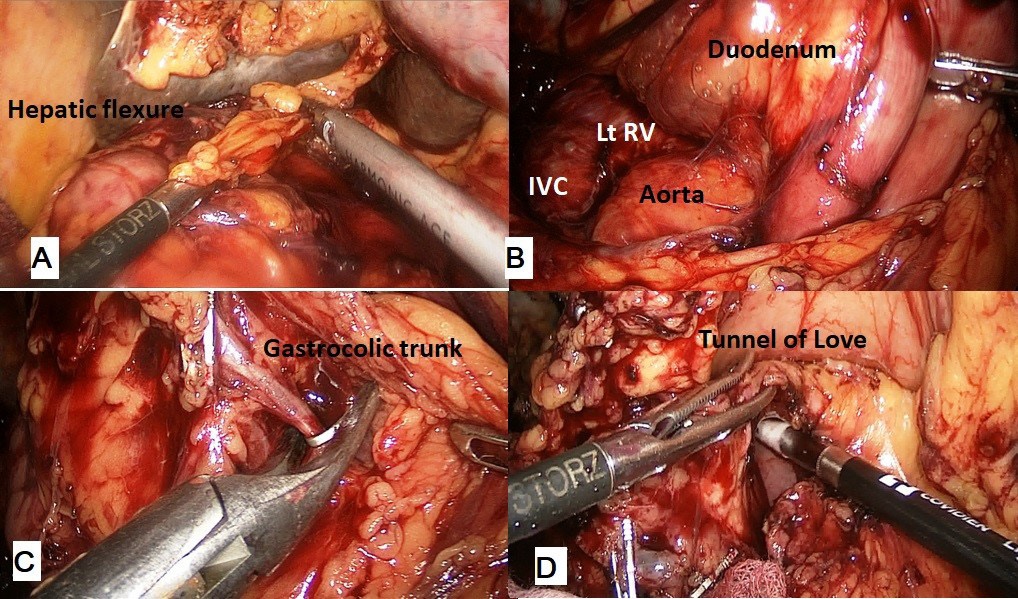
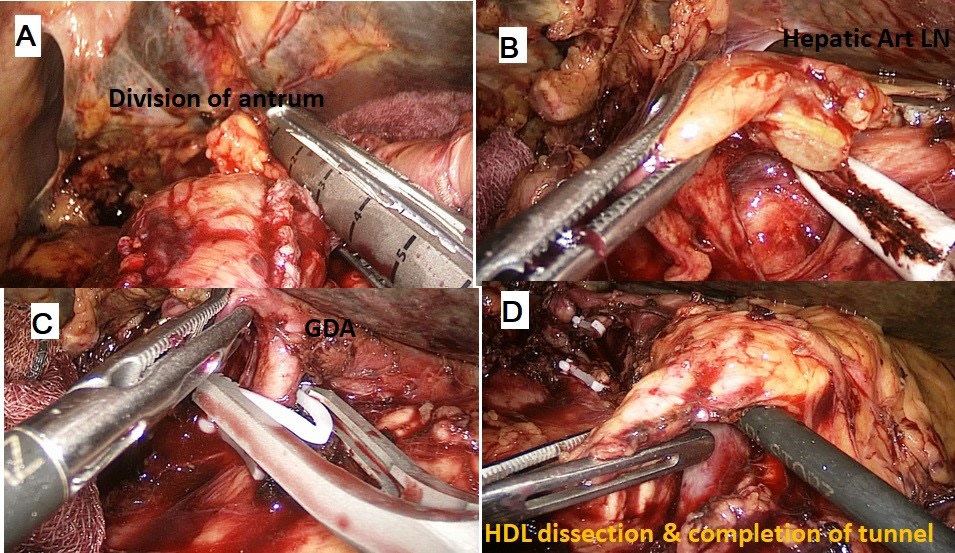
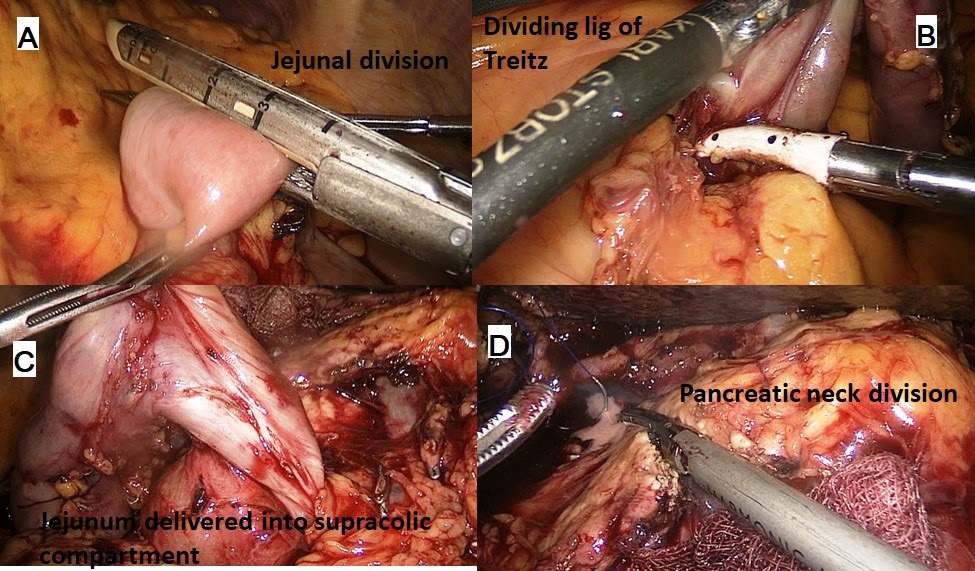
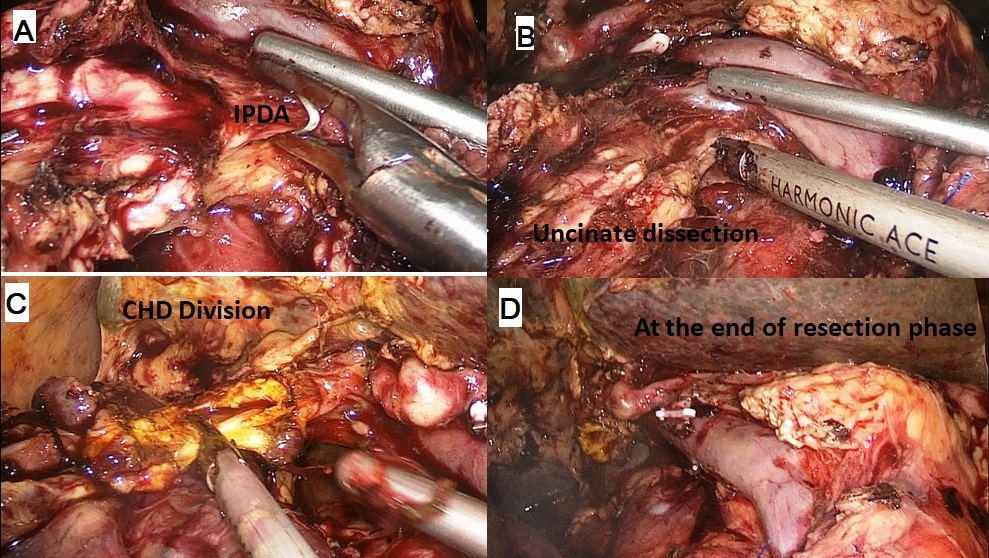
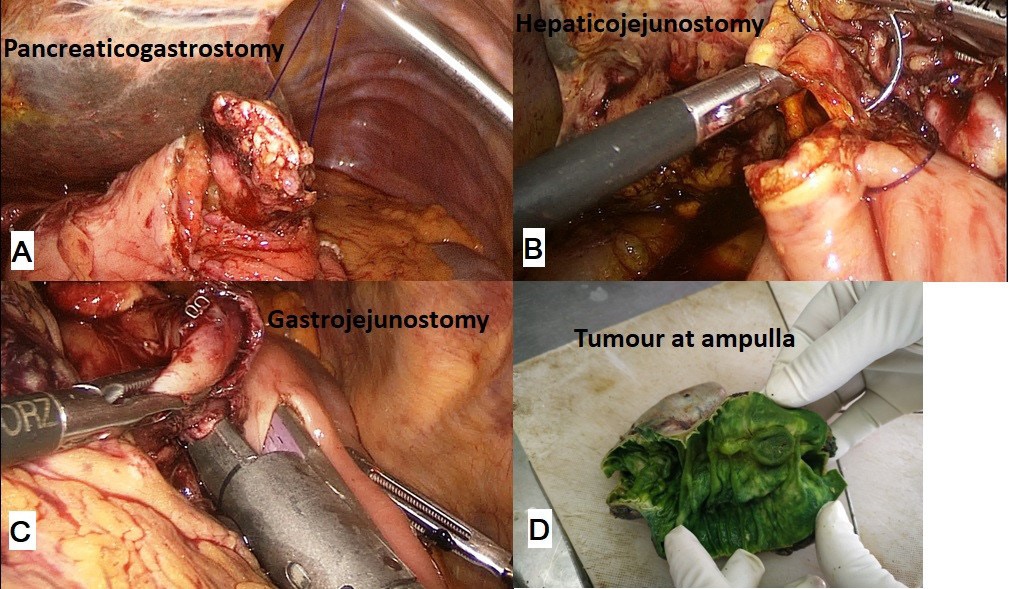
The procedure was performed with the patient in the French position and in a reverse Trendelenburg position. Typically, 5 ports were used for the procedure with the 6th (epigastric) port being optional. After establishing a pneumoperitoneum, a thorough diagnostic laparoscopy to rule out any dissemination was done. The energy devices used were a bipolar vessel sealing device (LigaSureTM, Medtronic, Minneapolis, USA) and a ultrasonic shear (Harmonic Ace®, Ethicon, Cincinnati, USA). The gastrocolic ligament was divided and the lesser sac entered. Next, the hepatic flexure of the colon was lowered and a wide Kocher maneuver was performed till the left renal vein was completely exposed (Figure 1A & 1B). The superior mesenteric vein was identified at the inferior border of the pancreas. The fusion fascia of Fredet was gently dissected to identify the gastro-colic trunk of Henle and the right gastroepiploic vein was ligated and divided. The ‘tunnel of Love’ was dissected posterior to the pancreatic neck, anterior to the superior mesenteric vein and portal vein (Figure 1C & 1D). The stomach was divided with a linear stapler at the level of antrum (Figure 2A). The hepatic artery lymph node was dissected to identify the common hepatic artery (Figure 2B). The gastroduodenal (Figure 2C) and right gastric arteries were then clipped, and divided. The complete packet of lymphnodes was taken along (Figure 2D). The cystic artery and duct were clipped and divided. The jejunum was transected 15 cm distal to the ligament of Treitz with the linear stapler and the fourth portion of the duodenum and proximal jejunum were mobilized (Figure 3A & 3B). The jejunal stump was then passed into the supracolic compartment (Figure 3C). The pancreatic neck was divided with the ultrasonic shear (Figure 3D). Dissection of the pancreatic head and uncinate process off the portal vein, superior mesenteric vein, and superior mesenteric artery was performed using the bipolar vessel sealing device. The inferior pancreaticoduodenal artery encountered during this dissection was clipped and divided (Figure 4A & 4B). All peripancreatic lymphatic tissue was taken en bloc with the specimen. The CBD was divided just above the junction with cystic duct (Figure 4C) and a bull dog clamp was applied to the common hepatic duct. A cholecystectomy was then performed. The specimen was then placed behind the liver and the resection bed was inspected and hemostasis ensured (Figure 4D). Now the reconstruction was started. The jejunum was brought through the duodenal resection bed, posterior to the superior mesenteric vessels into the supracolic compartment. Depending on the texture of pancreas and the duct size, either a pancreaticojejunostomy (PJ) or a pancreaticogastrostomy (PG) was done. The PJ done was end-to-side, duct-to-mucosa anastomosis performed over an 8 cm Silastic tube with an inner layer of 5-polydiaxonone (PDS) sutures and an outer layer of continuous 3-0 silk sutures. The PG was done end to side, using the ‘dunking’ technique (Figure 5A). Approximately 10 cm distal to the PE, an end-toside hepaticojejunostomy (HJ) was performed with running 3-0 barbed PDS sutures (Figure 5B). Approximately 40 cm distal to the HJ, a stapled antecolic, Gastrojejunostomy (GJ) was performed (Figure 5C). A single tube drain was placed behind the HJ. The specimen was then removed in an endobag though the umbilical trocar site extended to about 5 cm. A feeding jejunostomy (FJ) was done approximately 20 cm distal to the GJ using the Witzel’s technique.
Perioperative care
Patients were initially managed in the Intensive care unit and then shifted to surgical ward subsequently when continuous monitoring was deemed not necessary. Analgesia consisted of diclofenac and paracetamol infusions with supplemental tramadol which was switched to oral analgesics once orals were started. Perioperative prophylactic antibiotics consisted of Cefaperazone/sulbactam, Amikacin and Metronidazole given at time of induction which was continued for 2 days after surgery. Deep venous thromobisis prophylaxis was started on the first postoperative day (POD) with low molecular weight heparin. Typically, on POD 1, the nasogastric tube was removed if the output was less than 300 ml. A clear liquid diet was begun on POD 3, and the diet was advanced as tolerated. Simultaneously FJ feeds were initiated and advanced over the next two days. Drain fluid amylase was measured on the 3rd POD and the drain was removed when the output was less than 50 ml/24 hr. Patients were discharged after when they were tolerating solid diet and had no signs or symptoms of complications.
Data collection
Preoperative data: We collected data regarding demographics, preoperative biliary drainage, comorbid conditions, imaging, and biopsy report if any.
Operative data: We collected information on operative findings particularly duct size, pancreatic texture (as determined by the operating surgeon), total operation time, blood loss, blood transfusion, conversion to open surgery, and the reason for the same.
Postoperative clinical data: Morbidities including pancreasspecific complications, wound complications, pulmonary complications, and mortality, if any, were recorded. The length of hospital stay was noted. The need for re-exploration and readmission was determined. The lymph node yield and percentage of R0 resections were recorded.
Definitions
LPD: LPD involved the entire procedure, resection, and reconstruction performed laparoscopically except for feeding jejunostomy, which was conducted following the extraction of the specimen through 5 cm mini-laparotomy through the umbilical port.
Conversion: Conversion from laparoscopic to open surgery was defined as any unplanned switch from laparoscopy to laparotomy for reasons other than specimen extraction.
Hybrid PD: A planned conversion where the entire resection was performed laparoscopically and the reconstruction was conducted through an approximately 8 cm mini-laparotomy
Morbidity: Procedure or non-procedure related complications requiring medical or surgical intervention.
Perioperative mortality: All deaths within 30 days of surgery or during the same admission, irrespective of the cause.
Postoperative pancreatic fistula: Postoperative pancreatic fistula (POPF) was defined in accordance with the definition of the International Study Group of Pancreatic Fistula [15].
Delayed gastric emptying: Delayed gastric emptying was defined based on the guidelines of the International Study Group for Pancreatic Surgery (ISGPS) [16].
Postpancreatectomy hemorrhage: This involved grade B or C post pancreatectomy hemorrhage as proposed by the ISGPS [17].
Results
During the study period, LPD was performed in 13 patients. Three cases had to be converted to open surgery: one due to bleeding during pancreatic stump mobilization for PG, one due to bleeding during uncinate dissection in the second case, and one due to unsatisfactory PG, which was converted and a redo PG was performed. All conversions were completed through minilaparotomy. Finally, only 10 patients underwent LPD.
Demographic and preoperative data
Among the 10 patients who successfully completed LPD, 6 were men; 6 and 4 had ASA grade II and III, respectively. The median age of the patients was 54 (range: 39–70) years, and the median BMI was 23.6 (range: 19.6–27.2) kg/m2. Four patients underwent PBD: 1 for cholangitis, 2 for bilirubin levels >20 mg/dL, and 1 because the patient deferred his surgery and a short metallic stent was placed in the waiting period. All the patients were operated for malignancy.
Intraoperative and postoperative data
A major proportion of the patients had a soft pancreatic texture with a small duct (90%), and PG was performed in all these patients except for one patient with a firm pancreas and a dilated duct in whom PJ was performed. Table 1 lists the details of intraoperative and postoperative outcomes.
Table 1: Intraoperative and postoperative data.
Variables |
Values |
Duration of Surgery* (Minutes) |
460 (430–550) |
Blood loss* (mL) |
480 (150–1000) |
Soft Pancreas/Small duct# |
09 (90) |
PG# |
09 (47.4) |
Blood transfusions* (Units) |
0 (0-5) |
LS* (Days) |
14 (7-30) |
Wound complications |
01 (10) [CD II] |
Pulmonary complications |
01 (10) [CD II] |
PF Grade B/C# |
03 (30) [CD II:1; III:1; V:1] |
DGE# |
01 (10) [CD II] |
PPH# |
01 (10) [CD III] |
Reoperations# |
02 (20) |
Readmissions# |
00 |
Mortality# |
01 (10) |
*Median (Range); # n (%); PG Pancreaticogastrostomy; LS Length of stay; PF Pancreatic fistula; DGE Delayed gastric emptying; PPH Post pancreatectomy hemorrhage, CD Clavein-Dindo grade
The overall complication rate was 40% with most of the events occurring in one patient with grade C POPF and was the only mortality in the series. A total of 2 reoperation were required: one due to a GJ leak that was managed laparoscopically and one due to PPH that was managed through the laparotomy suture ligation of the GDA stump.
Histopathology and early oncological outcomes
All the patients had malignancy. A total of 100% R0 resections were noted, and a satisfactory lymph node yield was achieved in 90% of the patients (Table 2).
Table 2: Histopathology and short-term oncological outcomes
Variables |
Values |
Tumor size (cm)* |
3 (1.5-5) |
Lymph node yield* |
18 (8-26) |
R0 resection# |
10 (100) |
Histopathology# |
02 (26.3) |
* Median (Range); # n (%)
Discussion
The first LPD was reported by Gagner and Pomp in 1994 [18]. Gagner reported their first series of 10 cases in 1997 in which they questioned the need for performing PD laparoscopically because of its high complication rate, long operative time, and no clinical benefits [19]. A literature review published in 2009 indicated that only 146 cases of LPD had been reported in the 15 years since the first case [20]. Subsequently, a large clinical series published by the Palanivelu group in 2007, followed by another one by the Kendricks group, have demonstrated satisfactory outcomes [21,22]. This led to increased interest in LPD in the last decade globally, and more than 20 studies compared LPD with open PD.
The goal of the application of laparoscopy to PD is not to reduce pancreas-specific complications. By contrast, the aim is to extend the benefits of MAS to PD in terms of blood loss, transfusion requirements, pain scores, intensive care unit and hospital stay, wound-related (SSI, wound dehiscence, and ventral hernia in the long term) and pulmonary complications, and functional recovery while demonstrating a non-inferiority in terms of pancreas-specific complications and oncological outcomes.
Palanivelu et al. (PLOT trial) and Poves et al. (PDAULAP trial) have reported a significantly shorter hospital stay (7 vs 13 days and 13.5 vs 17 days, respectively) [11,12]. The PADULAP trial indicated lower overall morbidity rates in the LPD arm and similar pancreas-specific complications and oncological outcomes in both the arms. The PLOT trial reported significantly lower blood loss in the LPD arm and similar outcomes in terms of complications and oncological safety in both the arms. The third RCT, the LEOPARD-2/3 trial [13], was prematurely stopped due to a higher mortality rate in the LPD arm (10% vs 2%). Although this trial had a robust methodology, it had flaws, especially with a 20% conversion rate and 22% of surgeries being graded below average in a video review by a panel of experts. Furthermore, two of the five deaths reported in the LPD arm were due to bowel ischemia following injury to the superior mesenteric vessels. The same group reported a mortality of 3.5% in 114 LPDs done prior to the start of this trial. A meta-analysis of these RCTs [10] indicated that LPD resulted in lower blood loss and comparable outcomes in terms of complications and hospital stay although the surgery duration was longer.
Conroy et al examined data from the National Cancer Database and reported that centers performing <20 LPD/year should be considered a LVC [23]. They observed that 82% of LPDs were performed at LVCs and higher mortality rates at such centres. In this series from a LVC, we noted that LPD is feasible and can be safely performed with diligent planning and audit (Table 3) compares our outcomes with those of studies conducted in high-volume centers reporting their experience of respective initial LPD case series. Most of these studies did not report their path to start LPD and do not indicate whether their initial cases were included in the analysis. We have included all patients who underwent LPD. Our conversion rate was 23%, and the most common reason for conversion was bleeding, which is also the common reason reported in the literature. In these patients, we completed surgery by making a small extension of the 5 cm incision that was required to extract the specimen in LPD.
Table 3: Initial LPD series (Minimum 10 completed LPD in the series).
Authors |
Dulucq*24 |
Palanivelu*21 |
Kendriks*22 |
Zureikat*25 |
Caruso26 |
Our series |
Year |
2006 |
2007 |
2010 |
2011 |
2017 |
2021 |
Patients |
13 |
42 |
65 |
14 |
10 |
13 |
Conversion (%) |
23 |
0 |
4.6 |
14 |
0 |
23 |
Blood loss (ml) |
107 |
65 |
240 |
300 |
220# |
480 |
Operative time (min) |
295 |
370 |
368 |
456 |
224# |
460 |
CR-POPF (%) |
8 |
7 |
18 |
36 |
10 |
30 |
Hospital stay (days) |
16 |
10 |
7 |
8 |
12.3# |
14 |
R0 section (%) |
100 |
100 |
89 |
100 |
100 |
100 |
Lymphnode harvested (n) |
18 |
13 |
15 |
18.5 |
24 |
18 |
Mortality (n) |
1 |
1 |
1 |
1 |
1 |
1 |
*Series from high volume centres
# These values are in mean; other authors have reported median values
CR-POPF Clinically relevant postoperative pancreatic fistula
Some institutions have a well-designed program for progression to MAS in PD. One such program is the one being followed at University of Pittsburgh Medical Centre (UPMC) for their robotic PD that is based on a five-step process involving simulator drills, biotissue drills, video-based learning, proctorship, and final review of the videos of robotic PD performed by an individual surgeon [27]. Zureikat et al. [25] described their experience of the initial 14 cases of LPD from the same institution; the surgeons practiced LDP on fresh frozen cadavers followed by some cases for whom they had a planned conversion before performing LPD. Surgeons at the Duke University hospital used a staged modular approach starting with the hybrid procedure and eventually performing LPD, which initially always involves two attending surgeons with a team approach [28]. One attending surgeon had expertise in minimally invasive foregut surgery, whereas the other was trained in open pancreatic surgery. We suggest the hybrid PD approach for LPD (our results of the same will be published elsewhere). We performed resection laparoscopically, and the more difficult reconstruction part was performed through mini-laparotomy of approximately 8 cm. The surgeon could decide whether to progress to LPDs or continue with the hybrid protocol depending on the experience and skill levels of the surgeon. Furthermore, if one can complete the resection phase in 240-300 minutes, a laparoscopic reconstruction may be attempted. However, if the resection takes a longer time, then it would be safer to perform the reconstruction through a mini-laparotomy. Some studies have suggested using the hybrid laparoscopy–robotic approach. We have taken a slightly different path to start performing LPDs. We extensively learned by watching videos. Furthermore, we split surgery into many small steps; these steps were essentially part of other laparoscopic surgeries including hemicolectomy, radical gastrectomy, radical cholecystectomy, choledochal cyst excision, and distal pancreatectomy. Thus, we utilized our experience gained while performing these surgeries and eventually applied it to LPD.
The strength of this study is that we included all patients who underwent LPD; thus, this study provides a true picture of our initial experience with LPD. In addition, we did not exclude any patient except if they had any suspicion of vascular involvement on imaging; thus, our study was not subjected to selection bias. This study has some limitations. Because of its retrospective nature, we do not have follow-up data on the time to functional recovery and start of adjuvant treatment. In addition, we did not perform a cost analysis that could have been difficult to calculate in our set up.
Declarations
Acknowledgements: None
Conflict of interest: There are no actual / potential conflicts of interest, financial or otherwise, relationships and activities to disclose.
Author contributions
AK: Conceptualization, Methodology, Data curation, Writing-Original draft preparation.
SK: Methodology, Data curation, Writing- Reviewing and Editing
References
- Changazi SH, Ahmed Q, Bhatti S, Siddique S, Abdul Raffay E, et al. Whipple Procedure: A Five-Year Clinical Experience in Tertiary Care Center. Cureus. 2020; 12: e11466.
- Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy-A comparative study. Int J Surg. 2012; 10: 475-9.
- Clancy TE, Ashley SW. Pancreaticoduodenectomy (Whipple operation). Surg Oncol Clin N Am. 2005; 14: 533-52.
- Sahora K, Morales-Oyarvide V, Thayer SP, Ferrone CR, Warshaw AL, et al. The effect of antecolic versus retrocolic reconstruction on delayed gastric emptying after classic non-pylorus-preserving pancreaticoduodenectomy. Am J Surg. 2015; 209: 1028-35.
- Kaistha S, Nandi B, Kumar A. Laparoscopic surgery in pancreatic diseases: Pushing the boundaries. Med J Armed Forces India. 2019; 75: 361-369.
- de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, et al. Dutch Pancreatic Cancer Group. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg. 2019; 269: 2-9.
- Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, et al. The learning curve in pancreatic surgery. Surgery. 2007; 141: 694-701.
- Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010; 145: 634-40.
- Fisher WE, Hodges SE, Wu MF, Hilsenbeck SG, Brunicardi FC. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg. 2012; 203: 684-90.
- Lin D, Yu Z, Chen X, Chen W, Zou Y, et al. Laparoscopic versus open pancreatoduodenectomy: a meta-analysis of randomized controlled trials. Rev Esp Enferm Dig. 2020; 112: 34-40.
- Palanivelu C, Senthilnathan P, Sabnis SC, Babu NS, Srivatsan Gurumurthy S, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017; 104: 1443-1450.
- Poves I, Burdío F, Morató O, Iglesias M, Radosevic A, et al. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg. 2018; 268: 731-739.
- van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, et al. Dutch Pancreatic Cancer Group. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019; 4: 199-207.
- Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015; 220: 831-8.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, et al. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138: 8-13.
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007; 142: 761-8.
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142: 20-5.
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994; 8: 408-10.
- Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg. 1997; 1: 20-5.
- Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009; 16: 726- 30.
- Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007; 205: 222-30.
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010; 145: 19-23.
- Conroy PC, Calthorpe L, Lin JA, Mohamedaly S, Kim A, et al. Determining Hospital Volume Threshold for Safety of Minimally Invasive Pancreaticoduodenectomy: A Contemporary Cutpoint Analysis. Ann Surg Oncol. 2022; 29: 1566-1574.
- Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006; 20: 1045-50.
- Zureikat AH, Breaux JA, Steel JL, Hughes SJ. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011; 15: 1151-7.
- Caruso F, Alessandri G, Cesana G, Castello G, Uccelli M, et al. Laparoscopic pancreaticoduodenectomy for tumors of the head of pancreas; 10 cases for a single center experience. Eur Rev Med Pharmacol Sci. 2017; 21: 3745-3753.
- Hogg ME, Besselink MG, Clavien PA, Fingerhut A, Jeyarajah DR, et al. Minimally Invasive Pancreatic Resection Organizing Committee. Training in Minimally Invasive Pancreatic Resections: a paradigm shift away from “See one, Do one, Teach one”. HPB (Oxford). 2017; 19: 234-245.
- Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, et al. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 2014; 21: 4014-9.