Japanese Journal of Gastroenterology Research
Review Article - Open Access, Volume 2
Ultrasonography in inflammatory bowel disease, current status and future prospects: A review on findings, diagnostic performance and ultrasound-based scoring systems
Pooneh Dehghan1; Hamed Norouzi2*; Shayan Zamani3; Mohsen Kaveh4; Mahyar Mohammadzadeh5; Amin Momeni Moghaddam6; Shahrokh Iravani7
1Associate Professor of Radiology; Imaging Department, Taleghani Hospital, Shahid Behesti University of Medical Sciences, Tehran, Iran.
2Department of Radiology, Taleghani Hospital, Shahid Beheshti University of Medical Sciences, School of Medicine, Tehran, Iran.
3Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran.
4Department of Radiology, Taleghani Hospital, Shahid Beheshti University of Medical Sciences, School of Medicine, Tehran, Iran.
5Department of Radiology, Taleghani Hospital, Shahid Beheshti University of Medical Sciences, School of Medicine, Tehran, Iran.
6Assistant Professor of Radiology, Taleghani Hospital, Shahid Beheshti University of Medical Science, Tehran, Iran.
7Gastroenterology and Hepatobiliary Research Center, AJA University of Medical Sciences, Tehran, Iran.
*Corresponding Author : Hamed Norouzi
Department of Radiology, Taleghani Hospital, Shahid
Beheshti University of Medical Sciences; School of
Medicine, Tehran, Iran.
Email: Hamednorouzi7337@gmail.com
Received : Apr 25, 2022
Accepted : Jun 15, 2022
Published : Jun 22, 2022
Archived : www.jjgastro.com
Copyright : © Norouzi H (2022).
Abstract
Introduction: Ultrasonography is considered as an accurate tool to diagnose Inflammatory Bowel Disease (IBD) and assess disease activity. However, studies about the accuracy of ultrasound, in particular about the severity and extent of the disease, are controversial. This review aimed to elaborate the current knowledge of ultrasound findings in IBD, evaluate its capability for diagnosis, describe ultrasoundbased scoring systems for disease activity and finally, identify gaps of knowledge as a bench research for future studies.
Methods: Search engines including Medline, Web of science and Scopus were searched by two independent investigators for eligible studies with relevant keywords in the English literature. Two researchers abstracted the systematically chosen manuscripts on structured forms. Finally, 30 articles were chosen for further review. Results: The prominent ultrasonic indicators for diagnosis and risk stratifying in IBD include bowel wall’s thickness, echotexture and elasticity, mesenteric fibro-fatty proliferation, lymphadenopathy, hyperemia and bowel wall inflammation seen on Color Doppler Ultrasound. A systematic review of the studies about the accuracy of ultrasonography in assessment of segmental involvement in IBD, showed a sensitivity of 76.1% to 92.7% and a specificity between 58.1% to 97.6%. The diagnostic performance was affected by disease zone, type (ulcerative colitis or Crohn’s disease) and body mass index. Identifying significant determinants for disease activity including bowel wall thickness, mesenteric inflammatory fat, and color Doppler signal (blood flow) have led to new scoring systems for IBD. Conclusion: Ultrasonography is an accurate, affordable and noninvasive method for evaluating IBD. Employment of new scoring systems for assessing disease activity has opened a novel perspective for early diagnosis and appropriate treatment. However, there is room for improvement in defining more accurate and objective Doppler indices with higher degrees of inter-observer agreement. Shear wave elastography also appears to be a promising technique in chronicfibrotic stages.Keywords: Ultrasonography; IBD; Crohn’s disease; Ulcerative colitis; Elastography; Ultrasonic diagnosis.
Abbreviations: IBD: Inflammatory Bowel Disease; CD: Crohn’s Disease; UC: Ulcerative Colitis; BMI: Body Mass Index.
Citation: Dehghan P, Norouzi H, Zamani S, Kaveh M, Mohammadzadeh M, et al. Ultrasonography in inflammatory bowel disease, current status and future prospects: A review on findings, diagnostic performance and ultrasound-based scoring systems. Japanese J Gastroenterol Res. 2022; 2(9): 1089.
Introduction
Inflammatory Bowel Disease (IBD) is a medical terminology for a group of maladies that cause chronic inflammation in the gastrointestinal tract and is categorized into two subtypes, including, Ulcerative colitis and Crohn’s disease. The etiology of these phenomena remains unclear, but it seems that various genetic, environmental and immunological factors may play a role in inciting the disease. The incidence of Crohn’s disease and ulcerative colitis in different geographical regions varies from 0.1 to 11 individuals for Crohn’s disease and from 0.5 to 24.5 individuals for ulcerative colitis per 100,000 people [1,2]. Based on previous studies, in recent years, the incidence of Crohn’s disease and ulcerative colitis has been increasing worldwide [3]. Due to better medical care in the recent years, the prevalence of IBD in high-incidence areas, doubles every 10 years. The disease reduces patients’ quality of life up to nearly 50% in some cases; this is mostly due to occurrence at a young age and the chronic nature of the disease [4,5]. Forgoing, it can be expected that IBD would become a more important health issue in the coming years; not only in the developed economies, but also in developing countries.
IBD is idiopathic; it is assumed that multiple factors contribute to the etiology of the disease. However, it seems that a dysregulated immune response to host’s intestinal microflora, might be an important etiology for the onset of the disease [6]. Based on the pathophysiology of IBD, inflammation of the intestinal mucosa, due to activation of different inflammatory mediators, plays an important role in onset and progression of both UC and CD. Such inflammatory pathways can be flared up by different etiological factors, including genetic variations and possible environmental factors. Gastrointestinal and extra-gastrointestinal complications should be a carefully monitored and noticed, because they can delay a timely diagnose the disease [7]. The definitive diagnosis of IBD is based on a multidimensional approach including physical examination, histological assessment as well as imaging. Because of the low specificity of clinical symptoms and simultaneous gastrointestinal and extragastrointestinal involvements, relying only on clinical symptoms and physical examinations can lead to major diagnostic errors and a delay in accurate diagnosis [8]. Several laboratory studies such as blood tests, inflammatory biomarkers, genetic markers, metabolite and enzyme levels and serological studies are employed to diagnose IBD [9]. Nonetheless, none of the laboratory evaluations has been proved to be sufficiently specific to place a firm diagnosis of IBD. In this regard, the role of imaging techniques is highlighted more than before and extensive research is being done to evaluate their efficiency. Basic imaging techniques such as upright chest and abdominal radiography, although nonspecific, have been helpful in early differential diagnosis of this disease [10]. Beside radiography, other imaging tools such as ultrasonography have been also employed to place an accurate diagnosis, however, studies on the accuracy and diagnostic sensitivity of these methods, and in particular in determining the severity and extent of the disease, have been associated with conflicting results [11,12]. This study aims, first, to better elaborate the importance of using ultrasound for diagnosis of IBD, and then proceeds with summarizing all the studies on the diagnostic performance of ultrasonography and its role in determining the severity of the disease and finally its scoring.
Material and methods
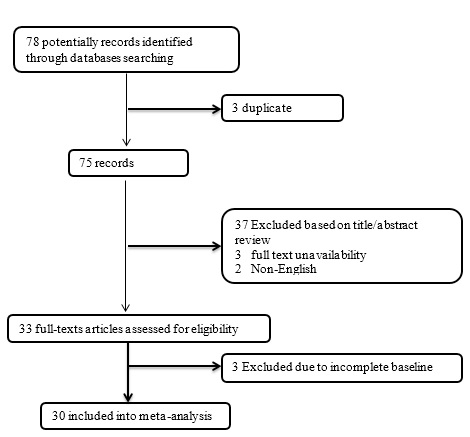
Firstly, two questions were suggested based on the authors purposes as “What is the role of ultrasonography in diagnosis of IBD and also stratifying its severity?” and “What are the main ultrasonic findings of IBD?” In the next step, the manuscript databases including Medline, Web of knowledge, and Scopus were thoroughly searched by two independent investigators for all eligible data using considered keywords including “inflammatory bowel disease”, “ultrasonography”, “accuracy”, and “risk score” by the end of 2020. The inclusion criteria were considered to retrieve the studies: 1) the selected studies were those that evaluated the role and diagnostic performance of ultrasonography in diagnosis of IBD, 2) The studies were restricted to English language, 3) the studies with unclear or irreproducible results were all excluded, 4) access to the manuscripts’ full texts was also considered in inclusion criteria unless the abstracts had enough data for our analysis, 5) case reports and case series papers were all excluded. Two of the participating researchers abstracted the selected manuscripts, un-blinded. Structured forms were applied to avoid any divergence in data gathering. Any disagreement among the reviewers were resolved by consulting a third reviewer or by reaching a shared accordance after discussion. The studies quality was evaluated based on the following criteria: 1) inclusion and exclusion criteria predefined in the studies as eligibility criteria; 2) searching the literature performed on a systematic and comprehensive approach; 3) to minimize the bias, the full texts of the article were reviewed by two researchers; 4) the quality of studies were evaluated and scored by the reviewers independently to assess internal validity; and 5) studies’ characteristics and findings were listed afterwards thoroughly. Any disagreement was resolved once again by discussion among the whole study team. After collecting complete information about the role of ultrasound in the diagnosis and scoring the level of disease risk, the final summary and analysis of the role of this tool was done and the value of its role was finally discussed. As shown in the flow diagram of the study selection structure (Figure 1), 78 manuscripts were initially collected by database searching. After removing 3 articles due to evidences of duplication, 75 records were primarily under-screened. Based on the titles and abstracts, 42 records were excluded and the remaining 33 citations were reviewed for additional eligibility. Out of those, 3 were excluded due to inconsistency of the data and incompleteness of contents. In final, 30 articles were found to be eligible for the final assessment [13-43].
Results
Ultrasonography-based findings in IBD
A variety of parameters have been identified for characterizing IBD and its-related inflammatory findings based on ultrasonography. One of the most prominent indices particularly for assessing the disease severity and activity is bowel wall thickness. It has been clearly shown that this anatomical marker is closely associated with clinical activity parameters including the Harvey Bradshaw index (HBI) and the CD activity index (CDAI) [13,14]. In different surveys, a vary of cut-off values have been defined for bowel wall thickening as the specific ultrasonic marker for IBD, however the cut-off value of 2 mm for the small intestine and 3-4 mm for the large intestine are universally agreed. It should be noted that increasing the cut-offs for disease diagnosis will reduce the diagnostic sensitivity of the instrument; so changing the cut-off value of 3 mm to 4 mm led to decreasing the diagnostic sensitivity from 88% to 75%, however, the specificity might increase from 93% to 97% [15]. Thus, although this marker could be very diagnostic, the standardization of its cutoff point had been an important limitation leading high interobserver disagreement (with kappa agreement values ranged 0.54 to 0.99) [16]. Another ultrasonic indicator for diagnosing and risk stratifying IBD is the echotexture of the bowel wall and its rigidity, while thickened wall can reflex inflammation activity. In this regard, although echotexture of the thickened bowel wall in patients with UC is mostly proportioned, disproportioning of the thickened bowel wall is usually visible in CD. Along with assessing echotexture of bowel wall thickening, the evaluation of the bowel surrounding structures, as a common finding in IBD is also facilitated by ultrasonography. Regarding this, discovering extramural changes within the mesenteric fat such as fibro-fatty proliferation (manifested by a hyperechoic zone surrounding the inflamed bowel) can be a critical sign of active inflammation. Another important ultrasonic feature, that indicates the severity of bowel inflammation in the background of IBD, is observing ascites as a result of IBD-related transmural inflammation [17]. Additionally, mesenteric lymphadenopathy can be well visible in ultrasonography views and is an important sign for disease activity particularly in patients suffering Crohn's disease [18]. Increased vascularization is also another ultrasonic indicator for severity of IBD that can be visualized with more sensitivity by color Doppler and contrast-enhanced ultrasonography and quantified by some scoring systems such as the Limberg score [19]. Importantly, Quantitative measurement of bowel wall vascularization correlates well with disease severity (indicated by endoscopic assessment), and thus, can be very helpful in predicting disease activity in IBD [20]. Bowel wall elasticity is another important imaging finding related to severity of IBD that can be assessed by Elastography. In fact, ultrasound elasticity imaging based on strain, the deformation and elastic moduli can distinguish low-grade fibrosis from highgrade fibrosis in intestinal tissue, based on their biochemical elastic properties.
Diagnostic performance of ultrasonography in IBD
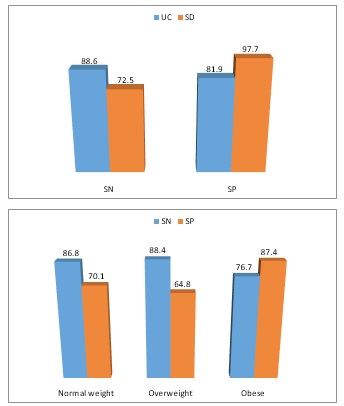
A variety of studies assessed the diagnostic performance of ultrasonography in assessment of IBD and its activity. Such studies can be divided into three subgroups including: 1) the studies focusing on the patient-based accuracy of ultrasonography in detecting segmental involvements in IBD among adults, 2) the studies aiming to compare the ability of ultrasound versus other diagnostic tools including surgery or endoscopy (toolbased) for the diagnosis of IBD and its Complications and 3) the studies assessing the patient-based diagnostic performance of ultrasonography in detecting IBD among children (Table 1). First, a systematically review of the studies on the accuracy of ultrasonography in assessment of segmental involvements in IBD (by considering bowel wall thickness >3 mm as the indicator for colorectal segments inflammation) showed a sensitivity ranged 76.1% to 92.7%, and a specificity ranged 58.1% to 97.6% for this tool [21-27] Interestingly, the diagnostic performance of ultrasonography was potentially affected by the assessed zones and segments so the pooled sensitivity and specificity of ultrasonography in detection of active inflammation in IBD were 75.5% and 96.6% in right colon, 79.7% and 93.9% in transverse colon, 94.2% and 78.6% in left colon, and 74.5% and 69.5% in rectum respectively. As another important point, the diagnostic performance was also affected by disease type (ulcerative colitis and crohn's disease) and patients’ body mass index (obese versus non-obese patients) (Figure 2). In this regard, first, the sensitivity and specificity of ultrasonography in ulcerative colitis subgroup was found to be 88.6% and 81.9% and in Crohn's disease subgroup was 72.5% and 97.7% respectively; indicating higher sensitivity in first subgroup and higher specificity in the latter group [28]. Secondly, it seems that ultrasonography for detecting IBD-related active inflammation is more sensitive among non-obese patients, but more specific in obese ones [28] (Figure 1).
As the second subgroups of studies, the diagnostic performance of ultrasonography in assessment of IBD was compared to standard references of endoscopic tools including ileo-colonoscopy or balloon enteroscopy [30-34]. Summing the results of these studies revealed a moderately high sensitivity and specificity of transabdominal ultrasonography in diagnosing IBD and its postoperative recurrence. Regarding these findings, sensitivity and specificity of transabdominal ultrasonography were yielded to be 54.0% to 93.3% and 97.0% to 100%, sensitivity and specificity of Doppler sonography were 67.0% and 80.0%, and sensitivity and specificity of contrast enhanced ultrasonography was shown to be 94.0% to 100% and 79.0% to 92.0% respectively (Table 2). In the pointed studies and with respect to detecting IBD-related complications, the diagnostic ability of ultrasonography devices was directly associated with such complications. In this regard, for detecting strictures, high sensitivity (79% to 97.5%), but a wide range of specificity (0% to 100%) were found for ultrasonography. Fistulas could be diagnosed with a sensitivity of 56% to 96% and a specificity of 88% to 100%. Also, abscess detection was achieved with a sensitivity ranged 89% to 100%, and a specificity ranged 80% to 95% [29].
Table 1: Diagnostic accuracy of ultrasonography in evaluation of colorectal segments in patients with IBD.
Author |
Right colon |
Transverse colon |
Left colon |
Rectum |
||||
SN |
SP |
SN |
SP |
SN |
SP |
SN |
SP |
|
Allocca et al (21) |
89.0% |
100% |
75.0% |
100% |
88.0% |
98.0% |
- |
- |
Allocca et al (22) |
44.0% |
100% |
29.0% |
98.0% |
89.0% |
88.0% |
- |
- |
Civitelli et al (23) |
74.0% |
100% |
86.0% |
100% |
95.0% |
100% |
- |
- |
Hashimoto et al (24) |
50.0% |
100% |
92.0% |
79.0% |
98.0% |
48.0% |
- |
- |
Kinoshita et al (25) |
82.0% |
84.0% |
81.0% |
79.0% |
86.0% |
49.0% |
90.0% |
30.0% |
Sagami et al (26) |
40.0% |
89.0% |
25.0% |
95.0% |
80.0% |
89.0% |
50.0% |
95.0% |
Sagami et al (27) |
90.0% |
95.0% |
100% |
79.0% |
100% |
46.0% |
59.0% |
56.0% |
Table 2: Diagnostic accuracy of ultrasonography compared to surgery or endoscopy in patients with IBD.
Author |
Index text |
Reference |
SN |
SP |
Borthne et al (30) |
TAUS |
IC |
93.3% |
98.8% |
Rispo et al (31) |
TAUS |
IC |
92.0% |
97.0% |
De Ridder et al (32) |
TAUS |
SBE |
54.0% |
100% |
Aloi et al (33) |
CEUS |
IC |
94.0% |
79.0% |
Horje et al (34) |
CEUS |
IC |
100% |
92.0% |
TAUS: Transabdominal Ultrasound; CEUS: Contrast Enhanced Ultrasound; IC: Ileocolonoscopy, SBE: Single Le Balloon Enteroscopy.
Ultrasound score for detection of IBD activity
Compared to standard tools such as endoscopic tools, some studies attempted to present new ultrasonic-based scoring systems to accurately detect IBD isolatable in children and adults. Among pediatrics, three ultrasonic parameters including bowel wall thickness, mesenteric fat, lymphadenopathy and hyperemia as seen on color Doppler signal were considered as the main components for this new scoring system. In this regard and using the ordinal logistic regression modeling, two sonographic parameters were identified as contributing significantly to disease activity including bowel wall thickness (labeled as absent, mild, moderate or severe) and visibility of mesenteric inflammatory fat and therefore, new scoring system (named the SPAUSS system) has been developed based on these two parameters. In this scoring system, for bowel wall thickness, a thickness range of 1.0 to 3.9 mm was scored 1, a thickness range of 4.0 to 6.9 mm was scored 4, and thicknesses greater than 7.0 mm were corresponded a score of 6. Qualitative descriptions of bowel hyperemia (Absent, mild, and moderate/severe) were assigned to scores of 0, 1, and 2, respectively for quantification. As for inflammatory fat, descriptive interpretations (absent, mild, and moderate/severe) were given scores of 0, 1, and 6, sequentially. By adding up all three scores a total score was obtained. Scores greater than 7 were resulted to be the most sensitive and specific for prediction for pediatric IBD [35].
In contrast, isolated scoring systems have been developed to predict IBD activity with the different diagnostic criteria. In this regard and in one of these systems presented by Novak et al [36] five ultrasound parameters including bowel wall thickness (BWT), color Doppler signal (blood flow), mesenteric inflammatory fat, mesenteric lymph nodes, and the presence of complications were considered as possible factors to be linked with disease activity, out of those, two parameters of bowel wall thickness and color Doppler signal were identified to have significant correlation with disease activity. In this regard, the following linear formulation was introduced to predict disease activity: Simple Sonographic Score = (0.0563 × BWT1) + (2.0047 × BWT2) + (3.0881 × BWT3) + (1.0204 × doppler1) + (1.5460 × doppler2). In another attempt by Ripollés et al [37], the sonographic findings determined as independent predictors for disease activity at endoscopy (SES-CD >3) included color Doppler grade, mural thickness, and contrast parameters (wash-in). In this regard, the equation applied for detection of endoscopic activity (SES-CD >3) is (parietal thickness × 0.957) +(color Doppler grade × 0.859) +(wash-in × 0.036). All pointed scoring systems could predict IBD severity with high sensitivity and specificity.
Endoscopic ultrasound elastography
Special attention has been paid to the role of endoscopic ultrasound (EUS) elastography in assessment and differentiation of different IBD phenotypes. In fact, this tool is very valuable in discrimination of stiffness of pathological and normal tissues in the regions affected by IBD [38]. EUS elastography was primarily inaugurated to distinguish malignant from benign pancreatic lesions, however this tool was gradually applicable for assessing any lesions with abnormal tissue stiffness due to inflammation, fibrosis, or necrosis or intestinal wall thickness changed due to such pathological changes. This technique cannot certainly replace biopsy or histological confirmation of the lesions nature or grade, although virtual biopsies carried out by EUS elastography can provide clinicians with useful information about the consistency of the affected tissue [39]. Additionally, strain elastography is a newly introduced sonographic technique than can possibly aid clinicians in diagnosis and follow up of IBD. This essay focused on demonstrating the feasibility of strain elastography in clinical setting by comparing visual observation and semiquantitative parameters with imaging studies obtained from endoscopic or other radiologic modalities in IBD patients [40].
Various recent studied attempted to compare the value of EUS elastography as another diagnostic tools for assessment of IBD pattern. In a study by Lo Re et al in 2017 and considering Entero-MRI as the reference, EUS elastography proved to be a useful tool for the evaluation of CD pattern [41]. Moreover, the value of EUS elastography in diagnosing and screening nonalcoholic fatty liver disease (NAFLD) in IBD patients has been demonstrated [42]. A number of scientists have focused on a new sonographic technique called shear-wave elastography (SWE), a modified method of elastography , to quantify tissue stiffness and differentiate inflammatory and fibrotic components in affected GI tissue in patients with IBD . Overall these studies have shown that SWE has a high sensitivity (87.5) and an acceptable specificity (57.9%) for distinguishing mild to moderate inflammations from sever inflammation [43]. Thus, it seems that SWE especially in combination with conventional sonography can be considered as an accurate diagnostic tool for detection of intestinal fibrosis among IBD patients.
Conclusion
Review of the literature shows acceptable sensitivity and specificity of ultrasonography as a method for detection of IBD and its activity status. Regarding the data, such diagnostic performance seems to be independent from patients’ age; and so both pediatrics and adults would benefit from this tool to prediction of IBD and evaluation of disease activity. However, it seems that the type of IBD (ulcerative colitis and Crohn's disease) as well as patients’ body mass index can affect the diagnostic accuracy of ultrasound in detection of IBD’s activity. Based on the critical parameters related to disease activity, particularly bowel wall thickness and color Doppler signal, new scoring systems can be designed specifically for pediatrics and adults for disease activity prediction. This hopefully would lead to a better patient management and therapeutic approaches for both groups. However, it should be noted that considering different references as the standard tool, study design, the baseline characteristics of study population, and especially involved bowel zones may potentially affect this diagnostic performance and thus may lead to significant heterogeneity.
References
- Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am. 2019; 99: 1051-1062.
- Wehkamp J, Götz M, Herrlinger K, Steurer W, Stange EF. Inflammatory Bowel Disease. Dtsch Arztebl Int. 2016 ; 113: 72-82.
- Lichtiger S. Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am. 2019; 29: 15-16.
- Lightdale CJ. Management of Inflammatory Bowel Disease: Progress and Promise. Gastrointest Endosc Clin N Am. 2019; 29: 13-14.
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014; 20: 91-9.
- Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am. 2019; 99: 1051-1062.
- Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019; 12: 113-122.
- Wright EK, Ding NS, Niewiadomski O. Management of inflammatory bowel disease. Med J Aust. 2018; 209: 318-323.
- Crowley E, Muise A. Inflammatory Bowel Disease: What Very Early Onset Disease Teaches Us. Gastroenterol Clin North Am. 2018; 47: 755-772.
- Olpin JD, Sjoberg BP, Stilwill SE, Jensen LE, Rezvani M, Shaaban AM. Beyond the Bowel: Extraintestinal Manifestations of Inflammatory Bowel Disease. Radiographics. 2017; 37: 1135-1160.
- Fraquelli M, Castiglione F, Calabrese E, Maconi G. Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: how to apply scientific evidence to clinical practice. Dig Liver Dis. 2020; 52: 9-18.
- Klett D. Sonographic detection and monitoring of inflammatory bowel disease. MMW Fortschr Med. 2019; 161: 61-63.
- Calabrese E, Petruzziello C, Onali S, et al. Severity of postoperative recurrence in Crohn’s disease: correlation between endoscopic and sonographic fi ndings. Infl amm Bowel Dis. 2009; 15: 1635-1642.
- Rigazio C, Ercole E, Laudi C, et al. Abdominal bowel ultrasound can predict the risk of surgery in Crohn’s disease: proposal of an ultrasonographic score. Scand J Gastroenterol 2009; 44: 585- 593.
- Fraquelli M, Colli A, Casazza G, et al. Role of US in detection ofCrohn disease: meta-analysis. Radiology 2005; 236: 95-101.
- Fraquelli M, Sarno A, Girelli C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis 2008; 40: 860-866.
- Baumgart DC, Müller HP, Grittner U, et al. US-based real-time elastography for the detection of fi brotic gut tissue in patients with stricturing Crohn disease. Radiology. 2015; 275: 889-899.
- Drews BH, Barth TF, Hänle MM, et al. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol. 2009; 19: 1379-1386.
- Limberg B, Osswald B. Diagnosis and diff erential diagnosis of ulcerative colitis and Crohn’s disease by hydrocolonic sonography. Am J Gastroenterol. 1994; 89: 1051-1057.
- Romanini L, Passamonti M, Navarria M, et al. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in infl ammatory bowel disease. Eur J Radiol. 2014; 83: 1317-1323.
- Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J Crohns Colitis. 2018; 12: 1280-1287.
- Allocca M, Fiorino G, Bonovas S, et al. Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis. 2018; 12: 1385-1391.
- Civitelli F, Di Nardo G, Nuti F, et al. Usefulness of colonic ultrasonography in the follow-up of pediatric ulcerative colitis: a prospective, blind, comparative study versus colonoscopy. Gastroenterology. 2014; 146: S-26.
- Hashimoto Y, Kume N, Sato K, et al. Development of a novel transabdominal ultrasound disease activity score in patients with ulcerative colitis (UCUS score). J Crohns Colitis. 2018; 12: S269.
- Kinoshita K, Katsurada T, Nishida M, et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterology. 2019; 54: 521-529.
- Sagami S, Kobayashi T, Okabayashi S, et al. Accuracy of Doppler transabdominal ultrasound in assessing disease severity and extent in IBD. J Crohns Colitis. 2019; 13: S218.
- Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther 2020.
- Shintaro Sagami, Taku Kobayashi, Yusuke Miyatani, Shinji Okabayashi, Hajime Yamazaki, Toshihiko Takada, et al. Accuracy of Ultrasound for Evaluation of Colorectal Segments in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis Clin Gastroenterol Hepatol. 2021; 19: 908-921.e6.
- Emma Calabrese, Torsten Kucharzik, Christian Maaser, Giovanni Maconi, Deike Strobel, Stephanie R Wilson, et al. Real-time Interobserver Agreement in Bowel Ultrasonography for Diagnostic Assessment in Patients With Crohn’s Disease: An International Multicenter Study. Inflamm Bowel Dis. 2018; 24: 2001-2006.
- Borthne AS, Abdelnoor M, Rugtveit J, et al. Bowel magnetic resonance imaging of pediatric patients with oral mannitol MRI compared to endoscopy and intestinal ultrasound. Eur Radiol. 2006; 16: 207-214.
- Rispo A, Imbriaco M, Celentano L, et al. Small bowel Crohn’s disease: comparison of enteroclysis, bowel sonography and Tc99m-HMPAO leukocyte scintigraphy. Eur Rev Med Pharmacol Sci. 2004; 8: 219-224.
- de Ridder L, Mensink PB, Lequin MH, et al. Single-balloon enteroscopy, magnetic resonance enterography, and abdominal US useful for evaluation of smallbowel disease in children with (suspected) Crohn’s disease. Gastrointest Endosc. 2012; 75: 87-94.
- Aloi M, Di Nardo G, Romano G, et al. Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric Crohn’s disease: a prospective, blinded, comparison study. Gastrointest Endosc. 2015; 81: 420-427.
- C S Horjus Talabur Horje, R Bruijnen, L Roovers, M J M Groenen, FBM Joosten, PJ Wahab. Contrast Enhanced Abdominal Ultrasound in the Assessment of Ileal Inflammation in Crohn’s Disease: A Comparison with MR Enterography. PLoS One. 2015; 10(8): e0136105.
- Amelia Kellar, Stephanie Wilson, Gilaad Kaplan, Jennifer DeBruyn, Divine Tanyingoh, Kerri L Novak. The Simple Pediatric Activity Ultrasound Score (SPAUSS) for the Accurate Detection of Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2019; 69: e1-e6.
- Kerri L Novak, Gilaad G Kaplan, Remo Panaccione, Elnaz Ehteshami Afshar, Divine Tanyingoh, Mark Swain, Amelia Kellar, Stephanie Wilson. A Simple Ultrasound Score for the Accurate Detection of Inflammatory Activity in Crohn’s Disease. Inflamm Bowel Dis. 2017; 23: 2001-2010.
- Tomás Ripollés, Joaquín Poza, Cristina Suarez Ferrer, María J Martínez-Pérez, Ana Martín-Algíbez, Berta de Las Heras Paez. Evaluation of Crohn’s Disease Activity: Development of an Ultrasound Score in a Multicenter Study. Inflamm Bowel Dis. 2021; 27: 145-154.
- Giovannini M. Thomas B. Erwan B. ChristianP .Fabrice C. Benjamin E. Genevieve M. Paolo A. Pierre D. Robert Y. 2009Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009; 1515871593.
- Gill KR, Wallace MB. EUS elastography for pancreatic mass lesions: between image and FNA? Gastrointest Endosc. 2008; 810951097.
- Andrea Giannetti, Marco Matergi, Marco Biscontri, Francesco Tedone, Lucia Falconi, et al. Strain elastography in Crohn’s disease: the role of visual observation and semiquantitative parameters. Journal of Ultrasound. 2019; 22: 227-239.
- Giuseppe Lo Re, Dario Picone, Federica Vernuccio, Laura Scopelliti, Ambra Di Piazza, et al. Comparison of US Strain Elastography and Entero-MRI to Typify the Mesenteric and Bowel Wall Changes during Crohn’s Disease: A Pilot Study. BioMed Research International. 2017; 4257987.
- Chiara Saroli Palumbo, Sophie Restellini, Che-Yung Chao, Achuthan Aruljothy, Carolyne Lemieux, et al. Screening for Nonalcoholic Fatty Liver Disease in Inflammatory Bowel Diseases: A Cohort Study Using Transient Elastography. Inflamm Bowel Dis. 2019; 25: 124-133.
- Yu-Jun Chen, Ren Mao, Xue-Hua Li, Qing-Hua Cao, Zhi-Hui Chen, Bao-Xian Liu, et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease. Inflamm Bowel Dis. 2018; 24: 2183- 2190.