Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 2
Spontaneous muscle hematoma in alcoholic liver cirrhosis: Uncommon but life-threatening complication
Volkanovska Nikolovska Anche*; Avramoski Vladimir; Andreevski Vladimir; Trajkovska Meri; Deriban Gjorgji
University Clinic for Gastroenterohepatology, Skopje, Republic of North Macedonia.
*Corresponding Author : Volkanovska N Anche
Institute of Pathology, Faculty of Medicine, Ss. Cyril and Methodius University in Skopje, North Macedonia.
Tel: +38970260789;
Email: ancevolkanovska@gmail.com
Received : Apr 26, 2022
Accepted : May 24, 2022
Published : Jun 03, 2022
Archived : www.jjgastro.com
Copyright : © Anche VN (2022).
Abstract
Spontaneous muscle hematoma (SMH) is encountered secondary to trauma or the presence of bleeding tendency due to hemophilia or anticoagulant therapy. Although not well-known, it is also a serious complication of liver cirrhosis and in this setting is associated with high mortality. Liver cirrhosis and alcohol abuse are independent causal factors for impaired hemostasis. Consequently, when these two conditions coexist the haemostasis imbalance is tipped toward bleeding. Since there is not established management of SMH in liver cirrhosis, the treatment of these patients is challenging. Various treatment modalities have been applied with different success. We report a case of SMH in a patient with longstanding alcohol consumption who presented with SMH of the muscles of the right thigh and anterior surface of the right calf. Liver cirrhosis was subsequently diagnosed. In the absence of major bleeding vessel and recurrent blood loss we decided for vigorous conservative treatment with the use of blood derivates and tranexamic acid. The patient was successfully stabilized and resolution of the hematoma ensued. A summary of the available literature is also given. In conclusion, SMH should be recognized by clinicians as a life-threatening complication in patients with liver cirrhosis.
Keywords: Spontaneous muscle hematoma; Alcohol abuse; Alcoholic liver cirrhosis; Tranexamic acid; Transcatheter arterial embolization.
Abbreviations: SMH: Spontaneous Muscle Hematoma; ALC: Alcoholic Liver Cirrhosis; AST: Aspartate Aminotransferase; INR: International Normalized Ratio; FFP: Fresh Frozen Plasma; RBC: Red Blood Cells; CT: Computed Tomography; TAE: Transcatheter Arterial Embolization; TXA: Tranexamic Acid.
Citation: Anche VN, Vladimir A, Vladimir A, Meri T, Gjorgji D. Spontaneous muscle hematoma in alcoholic liver cirrhosis: Uncommon but life-threatening complication. Japanese J Gastroenterol Res. 2022; 2(8): 1085.
Introduction
Spontaneous muscle hematoma (SMH) is typically seen in patients with coagulation disorders or in patients on chronic anticoagulation therapy [1]. Alcohol consumption is considered an independent cause of impaired hemostasis through variety of underlying mechanisms [2]. Alcohol is also known as the second most common cause of liver cirrhosis [3]. Liver cirrhosis is characterized by several coagulation alterations which, by itself, can be both pro- and antihemostatic [4]. SMH in patients with alcoholic liver cirrhosis (ALC) is a rare and not well described condition as only small number of isolated cases have been reported in the literature. However, the limited available data suggests that patients with ALC who develop SMH have very high mortality and early recognition with intensive treatment were implicated as crucially important for improved clinical outcome. Therefore, additional clinical reference is largely needed in order to recognize and better understand this rare but potentially fatal condition. This report describes a case of SMH in a patient concomitantly diagnosed with ALC and summarizes the available literature on this topic.
Case report
A 55-year-old male was admitted to a local hospital after developing increasing swelling and pain in the right knee over 3 days, associated with bluish-black discoloration of the overlying skin. Within 6 hours of the initial admission, he was transferred to the University Hospital in Skopje due to progressive extension of the swelling and skin discoloration, diffusely over the anterior portion of his right leg. The patient had no preceding trauma and was not using anticoagulation or antiplatelet therapy. He reported no history of easy bruising, epistaxis or visible blood in his stool and urine. He had no known medical history or previous surgeries. His family history was negative for any bleeding disorder. On social history, the patient reported daily alcohol consumption of 7 liquor shots over 10 years (approximately 65 grams of alcohol daily). At admission, the patient was tachycardic (pulse 115/min) but had normal blood pressure (120/70 mmHg), respiratory rate (15 breaths per min) and temperature (36.9°C). On general examination, he was ill-appearing, pale, and had mild diffuse icterus. Systemic examination revealed no abdominal organomegaly or stigmata of chronic liver disease, except mild jaundice. However, right leg examination revealed large, tense ecchymosis, extending diffusely over the anterior portion, between mid-thigh and the ankle. Distal pulses and sensory-motor function were preserved. Initial laboratory tests (Table 1) revealed profound anemia (hemoglobin 6.5 mg/dL, hematocrit 19%, mean corpuscular volume 98.4 fL) and mild thrombocytopenia (platelet count 141 X 109 /L). Comprehensive metabolic panel was significant for slightly elevated AST (75 U/L), predominantly indirect hyperbilirubinemia (73 μmol/L) and hypoalbuminemia (23 g/L). Coagulation studies showed prolonged prothrombin time (25.2 seconds) and increased INR (2). The patient was admitted in the surgical department. After admission he was transfused with 2 units of fresh frozen plasma (FFP) and 3 units of packed red blood cells (RBC).
Table 1: Initial laboratory values upon admission of the patient in the surgical department.
Parameter |
Result |
Normal range |
Parameter |
Result |
Normal range |
Hgb |
65 |
120 – 160 g/L |
PT |
25.2 |
12 s |
RBC |
1.93 |
4.5 – 5.5 x 1012/L |
aPTT |
46.8 |
30 s |
MCV |
98.4 |
84 – 98.0 fL |
INR |
2 |
1 |
Hct |
19 |
34 – 53.9 % |
D-dimers |
> 1000 |
< 500 ng/mL |
PLT |
141 |
150 – 400 x 109/L |
Total Bilirubin |
112 |
< 20 μmol/L |
WBC |
7.6 |
4.00 – 10.00 x 109/L |
Direct Bilirubin |
39 |
< 6.8 20 μmol/L |
|
|
|
Indirect Bilirubin |
73 |
< 13.6 μmol/L |
AST |
75 |
< 40 U/L |
Total protein |
55 |
63 – 83 g/L |
ALT |
30 |
< 56 U/L |
Albumin |
23 |
35 – 50 g/L |
Table 2: Reported cases of SMH in patients with alcoholic liver disease.
Case No. |
First author |
Age |
Sex |
Hematoma localization |
Treatment |
Outcome |
1 |
Docherty [12] |
48 |
Female |
Rectus abdominis |
unknown |
died |
2 |
Kamura [13] |
60 |
Male |
Ileopsoas |
conservative |
died |
3 |
Ishihara [11] |
59 |
Male |
Rectus abdominis |
TAE |
alive |
4 |
Yoshida [14] |
56 |
Male |
Rectus abdominis |
conservative |
alive |
5 |
Yoshida [14] |
62 |
Male |
Ileopsoas |
TAE |
died |
6 |
Hiraoka [15] |
56 |
Male |
Ileopsoas |
conservative |
died |
7 |
Hama [7] |
38 |
Female |
Ileopsoas |
TAE |
died |
7 |
Di Bisceglie [16] |
46 |
Female |
Rectus abdominis and retroperitoneal bleed |
conservative |
died |
8 |
Tozawa [17] |
60 |
Male |
Gluteus, biceps femoris and pectoralis |
conservative |
died |
9 |
Lee [8] |
47 |
Male |
Lateral thoracic artery |
TAE |
died |
10 |
Takasu [18] |
48 |
Male |
- |
conservative |
alive |
11 |
Sugiyama [19] |
56 |
Male |
Ileopsoas bilateral |
conservative |
died |
12 |
Parente [20] |
55 |
Male |
Ileopsoas |
TAE |
died |
13 |
Yamashita [10] |
60 |
Male |
Ileopsoas |
operation |
died |
14 |
Fakhrejahani [21] |
61 |
Male |
Rectus abdominis and Ileopsoas |
conservative |
alive |
15 |
Zacharia [22] |
50 |
Male |
Ileopsoas billateral |
conservative |
died |
16 |
Zacharia [22] |
54 |
Male |
Internal oblique muscle |
conservative |
died |
17 |
Zacharia [22] |
50 |
Male |
Intermuscular plane of the posterior left thigh |
conservative |
alive |
18 |
Lew [23] |
52 |
Male |
Ileopsoas |
TAE |
died |
19 |
Lew [23] |
41 |
Female |
Rectus abdominis |
TAE |
died |
20 |
Lew [23] |
55 |
Male |
Gluteus |
TAE |
died |
21 |
Takamura [9] |
50 |
Female |
Rightsided retroperitoneum |
TAE |
died |
22 |
Takamura [9] |
44 |
Male |
Muscles of left back |
conservative |
died |
23 |
Mangla [24] |
39 |
Male |
Gastrocnemius |
conservative |
alive |
24 |
Nhinda [25] |
39 |
Male |
Right gastrocnemius |
conservative |
alive |
25 |
Present case |
55 |
Male |
Right quadriceps and anterior surface of the right calf |
conservative |
alive |
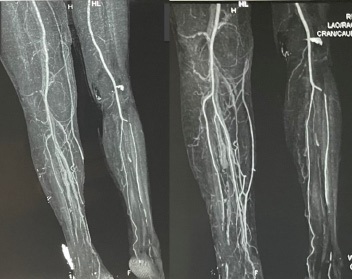
The following day the patient was sent to computed tomography (CT) angiography of the lower extremities. The CT angiogram (Figure 1) showed normal, patent vascular structures without contrast media extravasation. Notable CT abnormalities were swelling of the thigh and multiple loculated fluid collections with air inclusions in the knee region. Additional findings were the presence of free intraabdominal fluid, enlarged cirrhotic liver with portal vein dilatation and splenomegaly. The diagnosis of decompensated liver cirrhosis with severely impaired coagulation was established and the patient was transferred to the Clinic for gastroenterohepatology. Further treatment at our clinic included continuation of transfusions with FFP and packed RBC as needed as well as initiation of standard supportive treatment for decompensated liver cirrhosis with nonselective β-blocker (carvedilol) and diuretic therapy. Assessment regarding the etiology of cirrhosis established alcohol as the causative agent. Despite treatment with blood derivates (7 RBC units and 6 FFP units since initial presentation), a drop-in hemoglobin was noted on several control laboratory analyzes, raising suspicion of ongoing bleeding. Follow-up coagulation profile confirmed persistence of severely impaired clotting ability. After consultation with the traumatologist and transfusiologist, tranexamic acid (tid 0.5 g), cryoprecipatate (10 doses/daily) and enoxaparine (bid 40 mg) were included in the treatment. In the following 5 days there were no signs of ongoing bleeding while laboratory results showed steady improvement in hemoglobin and hematocrit level. The swelling of the right knee and the bluish-black discoloration slowly withdrew. Screening gastroscopy was performed and small straight esophageal varices were detected. After 16 days of hospital treatment the patient was discharged in stable condition. The patient was recommended a complete alcohol abstinence and regular follow up at our outpatient clinic. Up till this moment the patient is in improved and compensated condition in terms of the cirrhosis.
Discussion
Muscle hematoma can occur as a consequence of trauma or it can occur spontaneously. Intramuscular spontaneous bleeding is a well-known complication of hemorrhagic diseases (e.g. hemophillia) and anticoagulant therapy [1]. Frequently affected sites of bleeding are the ileopsoas and rectus muscle. When a bleeding diathesis is present intramuscular bleeding can occur even after trivial event, such as a cough-induced rise in intraabdominal pressure [1,5]. The occurrence of SMH in liver cirrhosis is a rarely reported event.
Liver cirrhosis is a condition characterized by multifactorial hemostatic defects. Main alterations that derange hemostasis in liver cirrhosis and promote bleeding diathesis are endothelial dysfunction and vasodilatation, altered endothelial-platelet interaction, decreased production of tissue factor pathway inhibitor, decreased production of factors involved in coagulation and decreased fibrinogen levels which is functionally aberrant due to excessive sialic acid [4]. Bleeding diathesis is also promoted by alcohol which is one of the leading causes for liver cirrhosis [3]. Alcohol has independent effect on coagulation through alterations in platelets function i.e. inhibiting platelet aggregation in response to collagen and adenosine diphosphate and partial activation of the platelets [2]. Both of these effects are thought to produce partially activated platelets which are incompletely granulated thus are not fully functional and poorly promote hemostasis. Additional mechanisms are thought to be a decrease in fibrinogen levels, von Willebrand factor and factor II and a decrease in fibrinolysis by increasing the tissue plasminogen activator activity [2]. The antihemostatic effect of both ALC and continuous excessive alcohol consumption potentiates when both conditions coexist. Thus, the aforementioned mechanisms could explain the occurrence of SMH in ALC.
From the literature review it is evident that bleeding in liver cirrhosis leading to SMH (Table 2) is not very frequently encountered as opposed to gastrointestinal bleeding and skin bleeds. Additionally, SMH in cases of cirrhosis has a serious prognosis that is quite different from the prognosis of SMH in patients with hemophilia or under anticoagulant therapy [1]. To our knowledge there are 25 reported cases of SMH in ALC in the literature including our patient. From the results of the reported cases of SMH in ALC it is apparent that this complication is associated with high mortality if not recognized and treated promptly. Therefore, early diagnosis is of crucial importance. Diagnosis of muscle hematoma can be obtained by ultrasound and CT, although contrast-enhanced CT has higher diagnostic sensitivity and specificity [6]. On contrast-enhanced CT, muscle hematomas appear as hyperdense lesions with their borders limited to the margins of the involved muscle/s. In attempt to identify a bleeding source CT angiography was performed in our case but the imaging study didn’t reveal one evident bleeding vessel. Additionally, emergency angiography has been also used as a diagnostic tool as shown in the published case reports, when it served to identify a bleeding source with intention to apply interventional treatment [7-9].
Interventional treatment includes radiological and surgical approaches. The literature review shows that out of 25 patients, only one patient underwent operation but did not survive [10]. Nine patients were subjected to transcatheter arterial embolization (TAE) out of whom only one survived [11]. Obstacles for successful radiological treatment could be the vascular fragility associated with liver cirrhosis and, at times the inability to identify a major bleeding vessel. Thus, it seems that surgical treatment and TAE do not offer any additional benefit in terms of survival.
Vigorous conservative treatment is preferred treatment option according to the data from the published case reports (Table 2) as well as from the experience with our patient. From the literature review it is evident that survival rate is highest in the group of conservatively managed patients. More specifically, as shown in table 2, out of 15 patients conservatively treated, 7 patients survived. It seems that prompt reversal of the coagulopathy with administration of FFP, vitamin K and/or cryoprecipitate increases the chance for survival. In our patient we also decided to use tranexamic acid (TXA) after initial failure to prevent ongoing bleeding with FFP substitution. TXA is an antifibrinolytic which prevents clot breakdown. Through occupying the lysine-binding sites on plasminogen and on circulating plasmin, TXA exerts inhibition of plasminogen activation and plasmin activity. TXA has a narrow scope indication mainly because of concerns about risk of thrombosis [26] which have made clinicians hesitant to use TXA. However, a recently published trial may change this perception as no evidence was found to the association of TXA and a higher risk of thrombotic complications [27]. Additionally, as recommended by the transfusiologist, cryoprecipitate and subcutaneous enoxaparine were also included. The combined use of TXA, cryoprecipitate and enoxaparine enabled us to stop effectively the ongoing bleeding with no thrombembolic complications and no need of further RBC transfusion.
Conclusion
It is evident that SMH in ALC is a very rare reported complication which occurs secondary to the coagulopathy promoted by the continuous alcohol consumption and the concurrent liver damage. Since it is a very rare complication, up till now there are no established guidelines for the treatment approach. SMH in the setting of ALC is associated with high mortality, consequently it is of utmost importance for clinicians to recognize it and treated it with no delay. Vigorous conservative treatment seems to be the best treatment modality while the role of TXA as an add-on to conservative treatment needs to be established.
References
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006; 85: 105-110.
- Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol. 2005; 123: S96-105.
- Singal AK, Anand BS. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis (Hoboken). 2013; 2: 53-56.
- Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009; 137: 2105-11.
- Dauty M, Sigaud M, Trossaërt M, Fressinaud E, Letenneur J, Dubois C. Iliopsoas hematoma in patients with hemophilia: a single-center study. Joint Bone Spine. 2007; 74: 179-83.
- Moreno Gallego A, Aguayo JL, Flores B, Soria T, Hernández Q, Ortiz S, González-Costea R, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg. 1997; 84: 1295-7.
- Hama Y, Iwasaki Y, Kawaguchi A. Spontaneous rupture of the lumbar artery. Intern Med 2004; 43: 759.
- Lee TH, Park YS, Chung DJ, Kim JH, Kim SM, Im EH, et al. Spontaneous rupture of the lateral thoracic artery in patients with liver cirrhosis. Korean J Intern Med. 2008; 23: 152-5.
- Takamura M, Watanabe J, Sakamaki A, Honda Y, Kamimura K, Tsuchiya A, et al. Alcoholic liver disease complicated by deep bleeding into the muscles or retroperitoneum: report of three cases and a review of the literature. Intern Med. 2014; 53: 1763- 8.
- Yamashita S, Tanaka N, Nomura Y, Miyahara T, Furuya T. Iliopsoas muscle hematoma secondary to alcoholic liver cirrhosis. Case Rep Gastroenterol. 2012; 6: 704-11.
- Ishihara Y, Nakae Y, Kanno T, Mukohbayashi C, Ikoma K, Nakazawa K, et al. A case of iliopsoas hematoma associated with liver cirrhosis, management by transcatheter arterial embolization. Shokakibyo Gakkai Zasshi. 2000; 97: 714-8.
- Docherty JG, Herrick AL. Bilateral rectus sheath haematoma complicating alcoholic liver disease. Br J Clin Pract. 1991; 45: 289.
- Kamura M, Tanahashi T, Yamakita N, Ikeda T: A case of idiopathic iliopsoas hematoma associated with liver cirrhosis. Nihon Shokakibyo Gakkai Zasshi 1998; 95: 1266-1269.
- Yoshida H, Tsuji K, Kawakami H, Katanuma A, Sakurai Y, JongHon K, et al. Two cases of alcoholic liver cirr hosis associated with intramuscular hematoma. Nihon Shokakibyo Gakkai Zasshi 2002; 99: 1350-1354.
- Hiraoka A, Michitaka K, Shigematsu S, et al: A case of alcoholic cirrhosis complicated with iliopsoas hematoma: difficulty in discriminating the diagnosis of progressive anemia. Kanzo 2004; 45: 609-613.
- Di Bisceglie AM, Richart JM. Spontaneous retroperitoneal and rectus muscle hemorrhage as a potentially lethal complication of cirrhosis. Liver Int. 2006; 26: 1291-3.
- Tozawa H, Kobayashi S, Muramatsu A, Hasegawa C, Hayakawa T. A case of alcoholic liver cirrhosis associated with intramuscular hematoma. Nihon Shokakibyo Gakkai Zasshi. 2006; 103: 839-43.
- Takasu O, Nakane T, Nakamura A, et al: A case of idiopathic iliopsoas hemorrhage in an alcohol abuser effectively treated with vitamin K therapy. J Jpn Assoc Acute Med. 2009; 20: 367-373.
- Sugiyama C, Akai A, Yamakita N, Ikeda T, Yasuda K. Muscle hematoma: a critically important complication of alcoholic liver cirrhosis. World J Gastroenterol. 2009; 15: 4457-60.
- Parente J, Siopa L. Hematoma Espontâneo do Ileopsoas: Uma Complicação Rara e Fatal da Cirrose Hepática [Spontaneous ileopsoas hematoma: a rare and lethal complication of liver cirrhosis]. Acta Med Port. 2012; 25: 55-7.
- Fakhrejahani F, Azar E G, Marnejon TP. Concurrent Rectus Sheath Hematoma and Iliopsoas Hematoma in a Cirrhotic Patient. J Blood Disorders Transf. 2013; 5: 184.
- Zacharia GS, Ray R, Sivaprasad P, Kolassery S, Ramachandran TM. Muscle hematomas: uncommon but horrendous complication of cirrhosis liver. Indian J Gastroenterol. 2014; 33: 289-91.
- Lew DH, Choi JY, Cha RR, Oh WH, Jo YW, Min HJ, et al. Three cases of spontaneous muscle hematoma in alcoholic liver cirrhosis. Korean J Med. 2014; 86: 472-477.
- Mangla A, Hamad H, Yadav U, Telfer M. Alcohol abuse and alcoholic liver cirrhosis leading to spontaneous muscle hematoma: an event fraught with danger. Case Rep Gastroenterol. 2015; 9: 93-100.
- Nhinda NT, Segamwenge IL, Nampweya T, Engels C. Spontaneous gastrocnemius muscle haematoma formation in a patient with alcoholic liver disease. Pan Afr Med J. 2020; 36: 348.
- Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011; 71: S9-14.
- Wang DS, Mazer CD, Orser BA. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N Engl J Med. 2017; 376: 1891-2.