Japanese Journal of Gastroenterology Research
Review Article - Open Access, Volume 2
Platelet-neutrophil crosstalk and neutrophil extracellular traps in acute pancreatitis
*Corresponding Author : Raed Madhi
Department of Biology, College of Science, University
of Misan, 62001 Maysan, Iraq.
Email: raed_saddam.madhi@med.lu.se
Received : Apr 10, 2022
Accepted : May 13, 2022
Published : May 19, 2022
Archived : www.jjgastro.com
Copyright : © Madhi R (2022).
Abstract
In addition to phagocytosis, cytokine production and degranulation, neutrophils utilize also another mechanism called NETosis in which neutrophils expel web-like structures called neutrophil extracellular traps (NETs). In this review, l focus on the role and mechanism of platelets in NETs formation in acute pancreatitis (AP). NETs involvement in the pathogenesis of AP is getting more appreciation and extra understanding in immune signaling and could provide potential therapeutic targets that might be a useful strategy to ameliorate local and systemic inflammation in (AP).
Keywords: Platelet; Neutrophil; Neutrophil extracellular traps; Integrins; Acute pancreatitis.
Abbreviations: AP: Acute Pancreatitis; NETs: Neutrophil Extracellular Traps; Intraperitoneal; ROS: Reactive Oxygen Species; MMP-9: Matrix Metalloproteinase-9; PSGL-1: P-Selectin Glycoprotein Ligand 1; IP6K1: Inositol Hexakis Kinase 1; PolyP: Platelet Polyphosphate; H3cit: Citrullinated Histone 3; MPO: Myeloperoxidase.
Citation: Madhi R. Platelet-neutrophil crosstalk and neutrophil extracellular traps in acute pancreatitis. Japanese J Gastroenterol Res. 2022; 2(8): 1084.
Introduction
Acute pancreatitis (AP) is an inflammatory disease that encountered in many countries with increased incidence [1,2]. Complications of AP are expected to elevate over time. In spite of improvement in diagnostic, several studies were observed that AP is associated with high mortality rate that ranging from 20% to 30% [1,3]. Since there is not specific treatment of AP, pancreatitis can continue to be connected with a serious morbidity and mortality. It is suggested that understanding pathophysiological mechanism of this disease might be contributed in resolve this problem. It is well known that the common cause of AP is gallstones that can obstruct the biliopancreatic duct and block the pancreatic secretions in side pancreas [4]. Subsequently, trypsinogen activation has reported to be the most common component in the pathogenesis of pancreatitis [5,6].
Indeed, it has shown that trypsinogen activation represents the early and transient process of onset of the pancreatitis [7]. Leukocytes recruitment is considered as a hallmark of inflammation in the inflamed pancreas and several studies have found that leukocytes play a critical role in the development of AP [8,9]. Beside their role in haemostasis and thrombosis, a recent study has shown that platelets are critical for neutrophil recruitment to sites of inflammation and infection [10].
Growing body of evidences has shown that activated neutrophils produce various inflammatory mediators such as neutrophil-derived reactive oxygen species (ROS), matrix metalloproteinase-9 (MMP-9) and elastase can be involved in the tissue damage in AP [11,12]. Activated neutrophils have been observed to use another mechanism called NETosis. It is process by which activated neutrophils release web-like structures consist of neutrophil-derived DNA decorated with nuclear histones, granular and cytoplasmic proteins, called neutrophil extracellular traps (NETs) [13,14]. NETs has recently shown to play a key role in in trypsin activation, neutrophil recruitment and tissue injury in AP [15]. In this review, l defines the essential role of platelets in neutrophils recruitment and NETs formation in AP.
Platelets dependent in neutrophil recruitment and activation
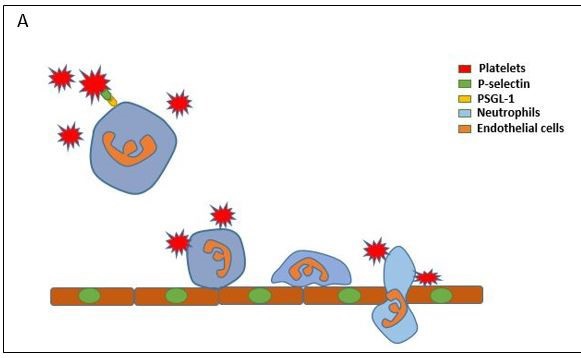
Platelets are small cells (2-3 µm) and unnucleated fragments that derived from megakaryocytes. Although they are not eukaryotic cells, platelets have mitochondria and endoplasmic reticulum residuals as well as they contain three different types of granules: alpha- granules, dense granules and lysosomes. These granules store variety of proteins and compound that have a critical role in both physiological and patho physiological conditions [16]. Having established that neutrophil infiltrated from circulation into inflamed and infected tissue in a gradual process with well-define sequential steps. Indeed, platelets have implicated to provoke all these steps from tethering to migration [10]. During inflammatory conditions, the adhesion molecules on the surface of platelets and endothelial cells are upregulated and that can led to slow down neutrophil movement in the circulation via forming a physical bridges providing a substantial adequacy of adhesion of neutrophils on the vascular endothelium [10] (Figure 1).
Beside these physical interaction, it has reported that activated platelets secrete various factors that additionally stimulate neutrophil adhesion as well as platelets also provoke adhesion molecule signaling that dependent in neutrophils and vascular endothelial cells interaction [17]. For example, previous studies have found that neutrophil recruitment to sites of inflammation substantially dependent on platelets through CD40L, CCL5 and CXCL4 secretion [18-21]. It is generally accepted that P-selectin consider the highly adhesion molecule for the interactions of platelet-neutrophil via binding with P-Selectin Glycoprotein Ligand 1 (PSGL-1) on the surface of neutrophil [22]. This concept was supported by other studies, for example, it has shown that using Ab direct against P-Selectin and PSGL-1 or use P-selectin deficient mice resulted in a significant reduction in neutrophilplatelets interaction [23-25] in AP [26]. Subsequently, another study was observed that interaction of P-selectin mediated neutrophil-platelets is essential for neutrophil interaction with vascular endothelium intravascular as well as neutrophil crawling and infiltration[25,27] (Figure 1).
Platelets-dependent NETosis
Platelet-neutrophil interaction have been observed to be substantially elevated during the inflammatory responses. For example, accumulating data have reported that platelet-neutrophil complexes are a substantial contributors to injury and acute inflammation in conditions such as abdominal sepsis [28], acute myocardial disease [29], pulmonary infections [30] and reperfusion injury [31].
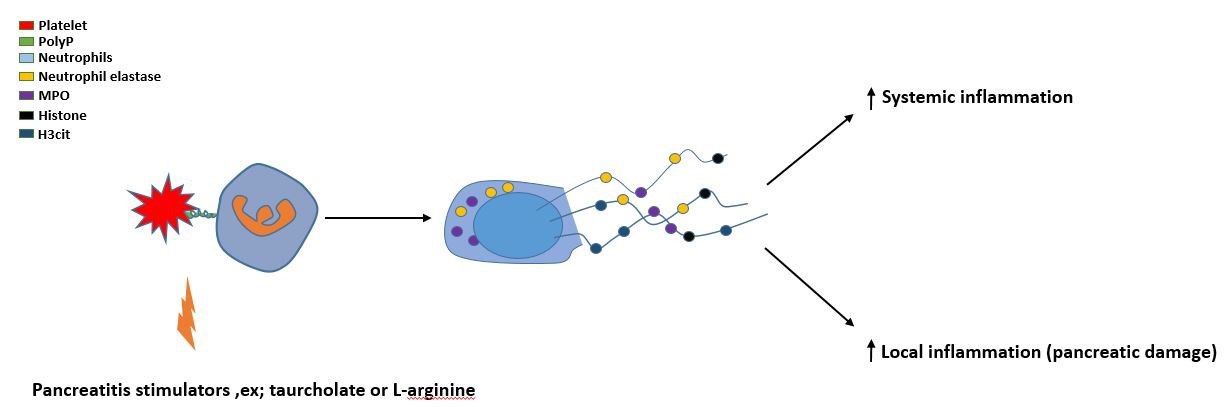
In fact, platelets are not only essential for neutrophil activation and recruitment, but they are also involved in induction of neutrophil extracellular traps (NETs). Structurally, NETs are composed of extracellular DNA decorated with nuclear and cytoplasmic proteins generating extracellular web-like structures [13,32]. It was recently shown that NETs provoke trypsin activation and inflammation in AP [33]. In addition, it was also observed that NETs cause a Ductal occlusion in AP [34]. A recent study has found that activated platelets induce NETs formation via TLR4 in enodotoxemia [35]. Moreover, it was reported that TLR-4 has a key role in neutrophil recruitment and tissue injury in AP [36]. Another study has observed that P-selectin is a critical for NETs generation [37]. Suggesting that these receptors provide a physical contact between platelet and neutrophil that is important for NETs generation. However, it is not clear how platelets provoke NETs formation. Luo HR et al, 2018 have found that platelet inositol hexakis kinase 1 (IP6K1) is a critical mediator for neutrophil activation and recruitment in lung during the systemic inflammation by forming platelet-neutrophil aggregation [38]. A recent study has established for the first time that platelet IP6K1, not neutrophil, regulates formation of NETs in AP [26]. In this study, IP6K1 knockout mice were used and it was found that NETs formation greatly reduced in IP6K1 deficiency mice compared with wild type mice. The authors suggested that IP6K1 might regulate NETs formation via controlling platelet polyphosphate (PolyP) (Figure 2).
It was found that the mixture of thrombin activation of IP6K1-deficent platelets and wild-type neutrophils in vitro led to a significant reduction in NETs formation compared with wild type group. Moreover, they observed that adding exogenous polyphosphate to the mixture of thrombin activation of IP6K1-deficent platelets and wild-type neutrophils was rescued NETs formation. Subsequently, the author indicated that platelet IP6K1 regulates NETs formation via PolyP secretion in AP. In addition, it has recently observed that activated platelets itself can bind with expelled NETs beside other molecules such as red blood cells, procoagulant molecules, extracellular vesicles and tissue factor. These complexes have found to enhance coagulation via activating the intrinsic pathway [39].
Key points
1. During inflammation conditions, platelets greatly involve to slowdown the movement of neutrophils in the blood stream.
2. P-selectin and PSGL-1 are the main mediators to provide a physical connection between platelets and neutrophils.
3. IP6K1 in platelets is required for neutrophil recruitment to the inflamed pancreatic tissue as well as for formation of NETs via regulating PolyP secretion.
Conclusion
In addition to their significant function in haemostasis and thrombosis, platelets play also a critical role in supporting of neutrophil recruitment to sites of inflammation through secretion variety of compounds such as CXCL4, CCL5 and CD40L. Moreover, activated platelets regulate neutrophil activation and NETs formation via a physical connection, represented by P-selectin and PSGL-1, and PolyP secretion that regulates by platelet IP6K1. This polymer of platelet phosphate could activate such a signaling pathways in the neutrophils that result in NETs formation in AP. It might be possible to utilize a therapeutic strategy In the future in order to target PolyP dependent neutrophil recruitment and NETs formation in the inflamed pancreas.
Acknowledgements: Raed Madhi is supported by Misan University, College of Science, Iraq.
References
- Munsell MA & Buscaglia JM. Acute pancreatitis. J Hosp Med. 2010; 5: 241-250.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993; 128: 586-590.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR & Windsor J. A. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010; 139: 813-820.
- Chatila AT, Bilal M & Guturu P. Evaluation and management of acute pancreatitis. World J Clin Cases. 2019; 7: 1006-1020.
- Wang GJ, Gao CF, Wei D, Wang C & Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009; 15: 1427-1430.
- Zhang XP, Li, Z J & Zhang J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009; 8: 351-357.
- Regner S, et al. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008; 8: 600-607.
- Abdulla A, Awla D, Thorlacius H & Regner S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011; 90: 975-982.
- Awla D, et al. Lymphocyte function antigen-1 regulates neutrophil recruitment and tissue damage in acute pancreatitis. Br J Pharmacol. 2011; 163: 413-423.
- Pitchford S, Pan D & Welch HC. Platelets in neutrophil recruitment to sites of inflammation. Curr Opin Hematol. 2017; 24: 23- 31.
- Wang HH, Tang AM, Chen L & Zhou MT. Potential of sivelestat in protection against severe acute pancreatitis-associated lung injury in rats. Exp Lung Res. 2012; 38: 445-452.
- Visweswaraiah SP. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis (Br J Surg 2012; 99: 246-255). Br J Surg 99, 1314; author reply. 2012; 1314-1315.
- Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004; 303: 1532-1535.
- Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007; 176: 231-241.
- Thorlacius K, et al. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol. 2006; 79: 923-931.
- McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001; 86: 746-756.
- Kolaczkowska E & Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013; 13: 159- 175.
- Rahman M, et al. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann Surg. 2009; 250: 783-790.
- Li G, et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am J Pathol. 2008; 172: 1141-1152.
- Hwaiz R, Rahman M, Syk I, Zhang E & Thorlacius H. Rac1-dependent secretion of platelet-derived CCL5 regulates neutrophil recruitment via activation of alveolar macrophages in septic lung injury. J Leukoc Biol. 2015; 97: 975-984.
- Wetterholm E, Linders J, Merza M, Regner S & Thorlacius H. Platelet-derived CXCL4 regulates neutrophil infiltration and tissue damage in severe acute pancreatitis. Transl Res. 2016; 176: 105-118.
- Larsen E, et al. Padgem Protein - a Receptor That Mediates the Interaction of Activated Platelets with Neutrophils and Monocytes. Cell. 1989; 59: 305-312.
- Kornerup KN, Salmon GP, Pitchford SC, Liu WL & Page CP. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol. 2010; 109: 758-767.
- Mayadas TN, Johnson RC, Rayburn H, Hynes RO & Wagner DD. Leukocyte Rolling and Extravasation Are Severely Compromised in P-Selectin-Deficient Mice. Cell. 1993; 74: 541-554.
- Sreeramkumar V, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014; 346: 1234-1238.
- Madhi R, et al. Platelet IP6K1 regulates neutrophil extracellular trap-microparticle complex formation in acute pancreatitis. JCI Insight, 2019; 4.
- Page G & Pitchford S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int Immunopharmacol. 2013; 17: 1176-1184.
- Salat A, et al. Changes of platelet surface antigens in patients suffering from abdominal septic shock. Thromb Res. 1999; 95: 289-294.
- Habazettl H, Hanusch P & Kupatt C. Effects of endothelium/leukocytes/platelet interaction on myocardial ischemia--reperfusion injury. Z Kardiol. 2000; 89: 92-95.
- Schaub RG, Rawlings CA & Keith JC. Platelet adhesion and myointimal proliferation in canine pulmonary arteries. Am J Pathol. 1981; 104: 13-22.
- Kohler D, et al. Phosphorylation of vasodilator-stimulated phosphoprotein (VASP) dampens hepatic ischemia-reperfusion injury. PLoS One. 2011; 6: e29494.
- Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. Journal of Cell Biology. 2007; 176: 231-241.
- Merza M, et al. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice With Severe Acute Pancreatitis. Gastroenterology. 2015; 149: 1920.
- Leppkes M, et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun, 2016; 7.
- Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007; 13: 463-469.
- Awla D, Abdulla A, Regner S & Thorlacius H. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflamm Res. 2011; 60: 1093-1098.
- Etulain J, et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015; 126: 242-246.
- Hou QM, et al. Inhibition of IP6K1 suppresses neutrophil-mediated pulmonary damage in bacterial pneumonia. Sci Transl Med, 2018; 10.
- Thalin C, Hisada Y, Lundstrom S, Mackman N & Wallen H. Neutrophil Extracellular Traps Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscl Throm Vas. 2019; 39: 1724-1738.