Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Serological and molecular detection to assess the prevalence of hepatitis A virus in Iranian patients with hepatitis manifestation
Sadeghi SA1,2; Rahimi P1,2; Bahramali G1,2; Sadat M2; Aghasadeghi MR1,2*; Hamidi-fard M1,2*
1Department of Viral Vaccine Research Center, Pasteur Institute of Iran, Alborz, Iran.
2Department of Hepatitis and AIDS, Pasteur Institute of Iran, Tehran, Iran.
*Corresponding Author : Mojtaba Hamidi-fard &
Mohammad Reza Aghasadeghi
Department of Hepatitis and AIDS, Pasteur Institute of
Iran, Tehran, Iran.
Tel: +98-21-64112240;
Email: mojtaba_hamidifard@yahoo.com &
mr_sadeqi@yahoo.com
Received : Apr 09, 2022
Accepted : May 09, 2022
Published : May 17, 2022
Archived : www.jjgastro.com
Copyright : © Hamidi-fard M & Aghasadeghi MR (2022).
Abstract
Background: Hepatitis A virus (HAV) transmits enterically and is highly endemic in developing countries. There are limited data on the prevalence of HAV in Iran; so this study was performed to detect HAV viral RNA in Iranian people.
Methods: Patients’ serums were collected from individuals with hepatitis symptoms in Tehran, Ahvaz, Shiraz, Kerman, Mashhad and Jiroft, Iran. The prevalence of anti-HAV IgM in the subjects was assessed by ELISA. The IgM positive samples were used for molecular detection of HAV Viral RNA using Real Time PCR.
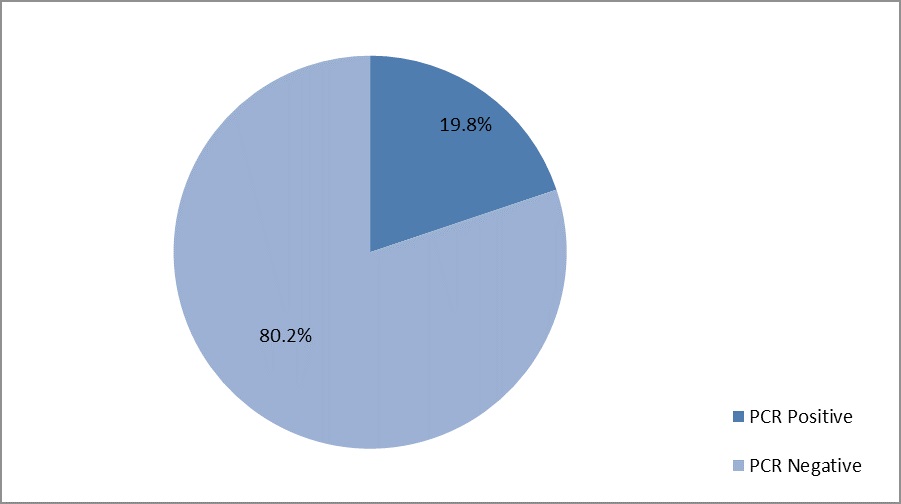
Results: According to the results of this study, the molecular prevalence of HAV was 19.8% amongst anti-HAV IgM positive patients. Moreover, the HAV infection was more prevalent in 19-21 years old people.
Conclusions: The results of this study highlight the fact that HAV is more prevalent in non-vaccinated people. What is more, the age of the onset of Hepatitis A has been raising which requires to be considered for vaccination and prevention planning.
Keywords: Hepatitis A; HAV prevalence; Real time PCR.
Citation: Sadeghi SA, Rahimi P, Bahramali G, Sadat M, Aghasadeghi MR, et al. Serological and molecular detection to assess the prevalence of hepatitis A virus in Iranian patients with hepatitis manifestation. Japanese J Gastroenterol Res. 2022; 2(7): 1082.
Introduction
Hepatitis A virus (HAV) is classified as Hepatovirus genus within Picornaviridae family [1]. It is a fecal-oral transmitting pathogen with a high outbreak in developing countries and areas of poor hygiene rather than developed countries. Epidemiologically, HAV divides into high, intermediate, and low endemic levels [2]. Hepatitis E Virus (HEV) transmits through contaminated water and foods as well. Both HAV and HEV are non-enveloped, positive sense and single-stranded RNA viruses with the RNA about 7.5 kb in length [3]. They can also gain pseudomembrane from hepatocytes while secreting into the blood [4]. HAV replicates in hepatocytes and causes liver malfunction.
It hurts hepatocytes by stimulating the host immune system. HAV includes seven genotypes (in which four genotypes affect humans) and one serotype [5].
The prevalence of HAV is strongly linked to income. Therefore, in low-income regions HAV emerges as a high endemic health threatening agent; although an improved hygiene system decreases its transmission probability [6]. The infection mostly occurs asymptomatically in children; from whom 70% have no symptoms and fulminant liver failure is scarce with no public health threatens. Nevertheless, it is important to work on HAV epidemiology and vaccination [7]. HAV infects children < 5 years asymptomatically in 90% of cases whereas about 70% of older children and adults infected with HAV represent acute hepatitis symptoms [8]. The risk of disease increases as getting older among communities [5]. Unlike the developing countries, adults in regions with rich socioeconomic conditions encounter a higher risk of morbidity and mortality. Millions of people are infected with HAV each year and also it led to about 11,000 deaths in 2015s [9]. Studies on Hepatitis A during 2016 - 2018 demonstrated a 294% increase compared with 2013-2015 [10].
HAV infection is a self-limiting disease and there is no effective medicine for it. HAV-infected people need weeks or months to recover from the illness in order to return to their daily life [11]. The immunity resulting from first exposure to HAV in childhood lasts many years [12]. According to the pre-exposured people in developing countries, clinical signs and symptoms from HAV are rare in these regions [4]. Seroepidemiological studies on HAV in Iranian populations of 10 - 18 years old from 16 provinces showed a higher prevalence of HAV people aged from 13 to 18 than the group between 10 - 12. According to previous seroepidemiological studies, more than 50 percent of Iranian people are immune to HAV infection [13].
In addition to the seroprevalence studies on HAV, RNA detection of HAV was done using PCR methods. In India, a molecular detection study showed that about 50 percent of seropositive samples (Anti- HAV IgM positive) were positive for HAV viral RNA [14]. A seroepidemiological and molecular prevalence study in Ahvaz, Iran showed that 28% of seropositive cases for HAV (42 of 150 samples) were PCR positive and contain HAV viral RNA [15].
Materials and methods
Population
In this cross-sectional population based study, we collected serum samples from patients with hepatitis sign and symptoms from Tehran, Ahvaz, Shiraz, Kerman, Mashhad and Jiroft, Iran with a sample size of 131 individuals aged between 6 and 30 years of both sexes. The informed consents were obtained from all patients before collecting the samples. This study was approved by the Ethics and Research Committee of the Pasteur Institute of Iran.
Sample collection
Ten ml of venous blood was obtained from each patient and sent immediately to the laboratory for the serum collection. The blood samples were centrifuged at 1500 g for 10 min to isolate serum from whole blood and all of the isolated serum samples were stored at -20˚C till the next procedures.
Serum sample analysis
The collected serum samples were tested for the detection of anti-HAV IgG and anti-HAV IgM antibodies using MyBioSource ELISA kit. The viral RNA was isolated by the QIAamp® Viral RNA kit according to the user manual. Furthermore, the HAV viral nucleic acid was assessed by the GeneProof Real-time PCR kit.
Statistical analysis
The data analyses were done using GraphPad Prism8.lnk (ver.8.0.2).
Results
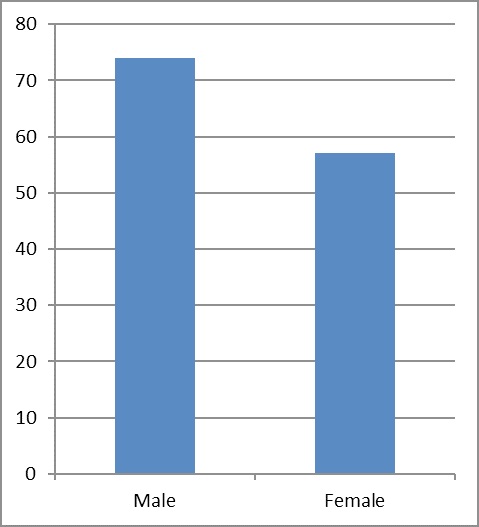
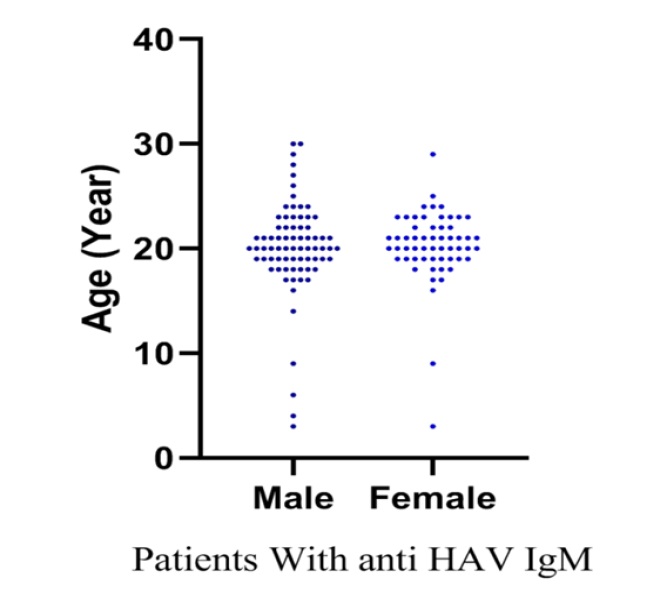
In this study a sample size of 131 patients with anti-HAV IgM were included; of these, 43.5% were men (Figure 1) and 50% aged from 19 to 21 years old (Figure 2).
Seroprevalence of hepatitis A
Of the total samples (131) used for detecting anti-HAV IgM by ELISA kit, anti-HAV IgM was detected in 98 serum samples (75%). The most seroprevalence of Hepatitis A was observed in the age group of 19-22 years old (Figure 2).
Molecular detection of hepatitis A virus
All of the IgM-positive samples were used for the detection of HAV viral RNA using Real time PCR method and the data showed that 19.8% of the patients with Hepatitis A were positive for HAV viral RNA and active infection (Figure 3).
Discussion
Iran is a country in the neighborhood of Iraq, Afghanistan and Pakistan in which according to the previous studies, the HAV prevalence was assessed as intermediate. A seroepidemiology statistics in 2014 stated an overall seroprevalence of 64% in which the lowest and highest prevalence of HAV was observed at age of 10 (14.8%) and 13 (72.9%), respectively [16].
In 2006 a seroprevalence study of HAV in 18-25 years old cases revealed 86% outbreaks with the following details; Tehran (65%), Hormozgan (92%), south, and north of Iran ( 99%) [7].
Different statistics were obtained from several studies carried out on HAV prevalence in Iran. This stems from the absence of any HAV seroprevalence program in Iran, where includes different regions along with various cultural and hygienic conditions [17]. In 2013, a statistical study on Shahrekord Center of Chaharmahal and Bakhtiari province population presented a HAV immunoglobulin G (IgG) prevalence of 90.8% (455 out of 501 serum samples). This prevalence was related to age, education level, marital status, and ethnicity [18]. From November 2011 to May 2012, 1030 serum samples were collected from the healthy population of Shiraz by a vast age range from 6 months to 95 years in those anti-HAV IgG and IgM were evaluated. The results indicated that 66.2% and 0.6% positive cases respectively [19].
Moreover, the other investigate was performed to assess anti-HAV IgG prevalence between March 2008 and March 2009. Consequently, among 1050 under-study subjects, 927 (88.2%) cases were reported positive and the rest of the population (123, 11.8%) reported as negative [20]. In 2012 a HAV seropositive of 32.9% was reported in a study carried out on 252 children who were admitted in the Gastroenterology Clinic of the Tabriz Children’s Hospital [21]. A vast region-based study on HAV seroprevalence was published in 2016 in which the 2562 serum samples were collected from students aged 10-18 years living in 27 provinces of Iran. The obtained ELISA results from Fars and Markazi provinces showed the lowest (50.43%) and the highest (78.81%) seroprevalence respectively [13].
This study showed that the age of infection with HAV has been raised which might stem from lack of HAV vaccination in children due to the fact that there is no specific treatment for HAV currently. Hence, it is crucial to vaccinate children against HAV in Iran which is also highly required in endemic area [22].
Conclusion
According to the results gained from this study, it seems that the people aged from 19-22 are more susceptible to Hepatitis A than other people. Therefore, a national vaccination program is required to be planned by national health organizations to prevent the progress of HAV infection in Iran.
References
- Bose M, Bose S, Saikia A, Medhi S, Deka M. Molecular epidemiology of hepatitis A virus infection in Northeast India. Journal of medical virology. 2015; 87: 1218-24.
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010; 28: 6653-7.
- Dogadov DI, Korzaya LI, Karlsen AA, Kyuregyan KK. Molecular genetic identification of isolates of the hepatitis A virus (HAV) from monkeys at Adler Primate Center. Journal of Medical Primatology. 2018; 47:87-92.
- Nelson KE, Kmush BL. Epidemiology of Viral Hepatitis A and E: A Global View. Viral Hepatitis in Children. Springer; 2019; 11-32.
- Organization WH. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review: Geneva: World Health Organization, 2010.
- Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harbor perspectives in medicine. 2018; 8: a031716.
- Merat S, Nouraie M, Abolghasemi H, Jamali R, Amini-Kafiabad S, Maghsudlu M et al. Seroprevalence and risk factors of hepatitis A virus infection in Iran: a population based study. Archives of Iranian medicine. 2010; 13: 99.
- Rezende G, Roque-Afonso AM, Samuel D, Gigou M, Nicand E, Ferre V et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003; 38: 613-8.
- World Health O. Global hepatitis report 2017: web annex A: estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2017.
- Foster MA, Hofmeister MG, Kupronis BA, Lin Y, Xia G-L, Yin S et al. Increase in hepatitis A virus infections—United States, 2013– 2018. Morbidity and Mortality Weekly Report. 2019; 68: 413.
- https://www.who.int/news-room/fact-sheets/detail/hepatitisa.
- Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 2006; 55: 1-CE-4.
- Mostafavi N, Kelishadi R, Kazemi E, Ataei B, Yaran M, Motlagh ME et al. Comparison of the prevalence and risk factors of hepatitis A in 10 to 18-year-old adolescents of sixteen Iranian provinces: the CASPIAN-III study. Hepat Mon. 2016; 16.
- Prakash S, Shukla S, Shukla R, Bhagat A, Srivastava SS, Jain A. Molecular characterization of hepatitis A virus circulating in Uttar Pradesh, India: A hospital-based study. Indian J Med Res. 2020; 151: 375-9.
- Tabasi M, Makvandi M, Teimoori A, Dastoorpoor M, Moradzadegan H, Parsanahad M et al. Epidemiology and Molecular Detection of HAV, HBV, and HCV in Patients with Acute Hepatitis Symptoms in Ahvaz. Jundishapur J Microbiol. 2018; 11: e63317.
- Hoseini S, Kelishadi R, Ataei B, Yaran M, Motlagh M, Ardalan G et al. Seroprevalence of hepatitis A in Iranian adolescents: is it time to introduce a vaccine? Epidemiology & Infection. 2016; 144: 291-6.
- Farajzadegan Z, Hoseini SG, Kelishadi R, Jamshidi F, Nokhodian Z, Noori R et al. Systematic review and meta-analysis on the agespecific seroprevalence of hepatitis A in Iran. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2014; 19: S56.
- Karimi A, Imani-Rastabi R, Moezzi M, Moradi MT. Hepatitis a seroprevalence and associated risk factors: A communitybased cross-sectional study in Shahrekord, Iran. Archives of Clinical Infectious Diseases. 2016; 11.
- Asaei S, Ziyaeyan M, Moeini M, Jamalidoust M, Behzadi MA. Seroprevalence of Hepatitis A and E Virus Infections Among Healthy Population in Shiraz, Southern Iran. Jundishapur J Microbiol. 2015; 8: e19311-e.
- Taghavi SA, Hosseini Asl MK, Talebzadeh M, Eshraghian A. Seroprevalence study of hepatitis A virus in Fars province, southern Iran. Hepat Mon. 2011; 11: 285-8.
- Rafeey M, Shoaran M. Prevalence and risk factors of hepatitis a in children in tabriz, iran. J Anal Res Clin Med. 2014; 2: 183-6.
- Herzog C, Van Herck K, Van Damme P. Hepatitis A vaccination and its immunological and epidemiological long-term effects–a review of the evidence. Human Vaccines & Immunotherapeutics. 2021; 17: 1496-519.