Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Clinical study of the efficacy of cTACE in combination with CalliSpheres® drug-loaded microsphere TACE in the treatment of large or giant hepatocellular carcinoma
Yu-Feng Wang; Jia Zeng; Hui-Wen Wang; Ying-Wen Hou; Jun-Rong Lu; Juan Wu; Dong-Feng He*
Harbin Medical University Cancer Hospital, Harbin 150081, Heilongjiang Province, China.
*Corresponding Author : Dong-Feng He
Research Scientist, Surgical Oncologist, Harbin Medical University Cancer Hospital, No. 150 Haping Street,
Nangang District, Harbin 150081, Heilongjiang Province, China.
Email: 13644578588@139.com
Received : Mar 23, 2022
Accepted : Apr 25, 2022
Published : Apr 28, 2022
Archived : www.jjgastro.com
Copyright : © Dong-Feng H (2022).
Abstract
Objective: CalliSpheres® microspheres (CSM) are the first drugeluting beads (DEB) developed in China. This study aimed to compare treatment response, survival, and safety profiles between conventional TACE (cTACE) in combination with DEB-TACE and cTACE in large or giant hepatocellular carcinoma (HCC) patients.
Methods: A total of 161 patients with HCC who underwent cTACE in combination with DEB-TACE or cTACE were included in this single-center retrospective study. Treatment response was assessed at third month (M3), and sixth month (M6) after TACE therapy; time to progression (TTP) and overall survival (OS) were evaluated; liver function indexes were recorded before TACE operation (M0), at first week (W1), at first month (M1) after TACE therapy; adverse events which occurred after TACE operation were recorded; Changes in AFP during treatment, the number of days of hospitalization, the number of treatments within 6 months and the treatment interval were analyzed.
Results: At 6 months after TACE, disease control rate was higher in combined group compared to cTACE (70.70% vs. 47. 60%, p=0.012<0.05). In contrast, the objective response rate at 3 months, objective response rate at 6 months, disease control rate at 3 months were similar in the combined group and the cTACE group, and the differences were not statistically significant (all p > 0.05). Regarding survival profiles, TTP [median: 16.080 months (95% CI: 11.636- 20.523) vs. 15.433 months (95% CI: 11.931-18.936)] as well as OS [median: 22.909 months (95% CI: 17.642-28.176) vs. 20.156 months (95% CI: 16.317-23.995)] were similar in combined group compared with cTACE group (both p>0.05). Multivariate Cox’s regression further illustrated that BCLC B vs C, Largest lesion diameter (cm) 5-10 vs >10, Number of lesions (n) <3 vs ≥3 were independent protective factors for OS (all p < 0.05). With regard to safety, patients in the cTACE group had slightly less impairment of liver function than those in the combination group. The incidence of pain and inflammation were lower in the cTACE group than in the combination group (p<0.05), while there was no significant difference in the occurrence of liver abscess, fever, or vomiting between the two groups. As for the number of days of hospitalization, the number of treatments within six months, there were no significant differences between the two groups. And the treatment interval (days) was longer in the combined group compared to the cTACE group55.000 (47.000 - 80.000) vs. 53.000 (46.000 - 62.000) p=0.014<0.05.
Conclusion: Compared to cTACE, cTACE combined with DEB-TACE for large or giant hepatocellular carcinoma shows a superior treatment response profile, and adverse effects were tolerated in both groups, with longer treatment intervals in the combined group.
Keywords: HCC; DEB-TACE; cTACE.
Abbreviations: ALPPS: Associating liver partition and portal vein ligation for staged hepatectomy; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AFP: Alpha-fetoprotein; BCLC: Barcelona liver cancer staging; CI: Confidence interval; CR: Complete response; cTACE: conventional Transcatheter arterialchemoembolization; DCR: Disease control rate; DEB: Drug eluting bead; DEB-TACE: Drug eluting bead Transcatheter arterialchemoembolization; HCC: Hepatocellular carcinoma; HR: Hazard ratio; HBV: Hepatitis B virus; HCV Hepatitis C virus; mRECIST Modified response evaluation criteria in solid tumours; ORR Objective response rate; OS: Overall survival; PR Partial response; PD: Disease progression; PT: Prothrombin time; PVTT: Portal vein tumor thrombus; SD: Stable disease; TACE: Transcatheter arterialchemoembolization; TTP: Time to progression; TBIL: Total bilirubin.
Citation: Yu-Feng W, Zeng J, Hui-Wen W, Ying-Wen H, Dong-Feng H, et al. Clinical study of the efficacy of cTACE in combination with CalliSpheres ® drug-loaded microsphere TACE in the treatment of large or giant hepatocellular carcinoma. Japanese J Gastroenterol Res. 2022; 2(6): 1077.
Introduction
Primary liver cancer is the sixth most common cancer in the world and the third leading cause of cancer deaths, and is on the rise every year, with China alone accounting for 50% of all liver cancer and tumour deaths worldwide [1]. Primary hepatocellular carcinoma includes 75-85% of hepatocellular carcinoma (HCC), 10-15% of intrahepatic cholangiocarcinoma (ICC), and mixed HCC-ICC. Liver transplantation, surgical resection and ablation therapy are the possible means to cure primary liver cancer, but they are only applicable to 20% of early stage patients. Due to the insidious biological characteristics of liver cancer, clinical symptoms and signs are not obvious. 60%-70% of patients are already in the intermediate and advanced stages when diagnosed, and the best time for surgery is lost, so the prognosis is not optimistic. The Barcelona liver cancer staging (BCLC) recommends transcatheter hepatic artery chemoembolization (TACE) as the first-line treatment for B stage HCC. In HCC patients with large tumor size [2], the tumor tissue often has multiple arteries involved in blood supply, with tortuous and thickened blood supply arteries, and with vascular invasion or arteriovenous fistulae. Treatment with drug-loaded microspheres (DEB) alone often requires more than one vial of DEB to reach the embolic endpoint; treatment with iodinated oil alone is equally large, and overdosing with iodinated oil can produce serious life-threatening adverse events. The Chinese primary Liver Cancer Treatment protocol (2021 Edition) states that the amount of iodine oil is usually 5-20 ml, not more than 30 ml. Super liquid iodinated oil can enter the hepatic sinusoids and peritumor portal vein micro-branches through the internal microvessels of tumor or peri-biliary plexus, blocking its blood supply to the marginal area of tumor. However, the diameter of the blood supplying arteries in the portal area of tumor is coarse, the flow rate is fast, and there are more fine arteries intertwined with blood supply in its root, so it is not easy to embolize these branches with conventional dose of iodinated oil, and the excessive dose is likely to cause reflux misembolism, while direct embolization of the main trunk of hepatic artery is very easy to form collateral circulation. And the diameter of iodinated oil is small, which is easily washed by blood flow and poorly deposited. At this time, additional DEB can effectively reduce the washout of iodinated oil by blood flow and mechanically occlude the blood supply to the tumor portal area, while continuously and slowly releasing chemotherapeutic drugs. The combination of the two can significantly reduce the dose used alone, increase the intensity and duration of tumor ischemic necrosis, and release chemotherapeutic drugs to the tumor in a controlled and sustained manner [3]. It has been noted [4-6] that cTACE had a very limited role when applied to large or giant hepatocellular carcinoma, with the ORR of only 16-29% and the mOS of only 6.5-9. 1m. In contrast, D-TACE for large or giant hepatocellular carcinoma achieved superior survival benefit, and in several studies [7-10], CalliSpheres drug-loaded microspheres TACE for large hepatocellular carcinoma showed good mOS of 11.5-16.0 m and mPFS of 6.6-7.5 m, demonstrating promising efficacy and tolerability. Therefore, the aim of this retrospective non-cohort study was to further compare the clinical value between cTACE in combination with Callispheres-loaded microsphere TACE and cTACE in large or giant HCC patients.
Methods
Study design and study endpoints
The aim of this retrospective study was to evaluate the immediate and long-term efficacy and adverse effects of comparing cTACE in combination with CSM-TACE with cTACE in the treatment of HCC patients. The primary evaluation criteria include objective response rate (ORR) and disease control rate (DCR) at 3 and 6 months; time to disease progression (TTP); overall survival time (OS); secondary evaluation criteria include postoperative adverse events; number of treatments required within 6 months; treatment interval between the first treatment and subsequent supplemental TACE; changes in AFP during treatment, length of hospital stay.
Patients
One hundred and sixty-one patients with HCC treated at the Harbin Medical University Cancer Hospital between January 2016 and January 2019 were included and followed up until November 2021 with a median follow-up time of 24.6 months (range: 4.2-51.6).The staging was performed with reference to the BCLC stage [11]. Large or giant HCC was defined as nodule size greater than 5cm or 10 cm in diameter [12].Inclusion criteria: 1. imaging and/or pathologically confirmed hepatocellular carcinoma; 2. single nodule or multiple fused nodules in diameter above 5 cm 3. patients (male or female) aged 18 to 75 years; 4. Child-pugh liver function rating: grade A; 5. medical records were kept complete and available for review; 6. laboratory indicators should meet the following criteria: (1) white blood cell count ≥ 3.0×109 /L (2) hemoglobin ≥ 8.5g/dl (3) platelet count ≥ 50 × 109 /L (4) glutamic aminotransferase (ALT) and glutamic aminotransferase (AST) are less than 3 times the upper limit of normal (5) albumin ≥3.0 g/dl (6) total bilirubin less than 3 times the upper limit of normal (7) prothrombin time international normalized ratio (INR) ≤2.3 or prothrombin time (PT) does not exceed the upper limit of normal control 3 seconds. (8) Serum creatinine less than 1.5 times the upper limit of normal Exclusion criteria: 1. Combination of primary malignancy at other sites; 2. Unstable systemic disease or uncontrolled infection; 3. patients who are pregnant or breastfeeding; 4. Chronic renal failure; 5. Child-pugh liver function rating: Grade C 6. Suffering from severe cardiovascular disease; 7. Severe coagulation dysfunction; 8. Hepatic encephalopathy, intractable ascites. The present study was approved by the Institutional Review Board of the Harbin Medical University Cancer Hospital, and written informed consents were obtained from all the patients or their statutory guardians.
Interventional procedures
Procedures of cTACE+DEB-TACE
The CSM (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) with diameters of 100–300 mm were used in the DEB-TACE procedure. Before DEB-TACE, the CSM were loaded with pirarubicin (THP) (40 mg)(Shenzhen Main Luck Pharmaceuticals Inc., China) , subsequently, the high concentration contrast agent was added into the CSM (loaded with THP) as 1:1 ratio, and then the mixture of contrast agent and CSM loaded with THP was kept still for 15 min for further use.
The patient was placed supine on the interventional table, the area was routinely disinfected and toweled, and the femoral artery was anesthetized with a local infiltration of 2% lidocaine 3 ml on both sides 1 cm below the right inguinal ligament. After successful puncture of the right femoral artery with a Seldinger puncture needle, a 5F catheter sheath was introduced and 20 ml of sodium heparin saline solution was injected through the catheter. A guide wire and a 4F hepatic artery catheter were introduced, the catheter was inserted into the aortic arch, the shape of the catheter was restored, and dexamethasone sodium phosphate 10 mg was slowly injected through the catheter. The catheters were selected into the phrenic artery, common hepatic artery, and superior mesenteric artery for imaging to assess the blood supply to the tumor and to see the tumor foci stained. The microcatheters were selected into the tumor blood supply vessels separately.
Suspension of ethiodized poppyseed oil injection (EPO), Lobaplatin for Injection (50 mg) and Raltitrexed for Injection (2 mg) were confected. After that, the confected iodized oil chemotherapy drug emulsion was injected into the tumor-supplying vessel. Subsequently, the mixture of CSM was injected at a speed of 1 ml/min until the flow of contrast agent stagnated. (Note: The amount of iodized oil was based on the diameter of multiple lesions and, for example, 5.5 cm in diameter, 5.5 ml of iodized oil, and 10 ml of iodized oil was also used for diameters larger than 10 cm) .Embolization stoped when blood flow slowed down and small branches of the portal vein were visualized or when waiting for 2-5 cardiac cycles for the contrast to not empty. The catheter and sheath are removed and local pressure is applied for 10 minutes to confirm that there is no bleeding from the incision and no subcutaneous haematoma. Local dressing with pressure and return to ward. Routine hepatoprotective, anti-infective and symptomatic treatment was administered.
Procedures of cTACE
Suspension of EPO,50 mg LBP,2 mg Raltitrexed and 20 mg THP were confected before cTACE. The processes of angiography and puncture of cTACE were performed as the same as DEB-TACE procedures. After that, the iodized oil chemotherapy drug emulsion was injected into the tumor-supplying vessel. Then, gelatin sponge particles with diameters of 150-300 mm(Hangzhou Aili Pharmaceutical Technology Co., Ltd., China) were added until the stenosis of the flow occurred. In addition, the angiography was performed for another time to detect if there was incomplete embolization.
Post-treatment follow-up and assessment of efficacy
Liver function indicators including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and albumin (ALB) were checked before TACE ,1 week after the procedure and 1 month after the procedure. Adverse effects after treatment were recorded, including liver abscess, fever, pain, and nausea and vomiting due to therapy. Enhanced computerized tomography (CT) or enhanced magnetic resonance imaging (MRI) was performed for treatment response assessment at third month (M3),and sixth month (M6) after therapy, and as for patients with deficient deposit of EPO, residual lesions, or recurrence, TACE was repeated. The lesions were assessed according to the modified efficacy evaluation criteria for solid tumors (mRECIST) and were classified as: complete response (CR), partial response (PR), disease progression (PD), stable disease (SD), objective response rate (ORR) of CR+PR and disease control rate (DCR) of CR+PR+SD, as shown in Table I. Time to progression (TTP) was defined as the duration from the time of first TACE operation to the time of disease progression. Overall survival (OS) was defined as the duration from the time of first TACE operation to the time of death. All patients received inpatient and telephone follow-up.
Table 1: Criteria for evaluating the efficacy of solid tumours (mRECIST).
Evaluation |
mRECIST |
|
|---|---|---|
Complete remission (CR) |
Loss of arterial phase enhancement in all target lesions |
|
partial Remission (PR) |
Combined reduction of ≥ 30% in the diameter of the target lesion (arterial phase enhancement) |
|
Disease progression (PD) |
Total increase in diameter of target lesions (arterial phase enhancement) ≥ 20% or new lesions |
|
Disease Stabilisation (SD) |
Reduce not to PR or increase not to PD |
|
Statistical analysis
All data were processed and analyzed using IBM SPSS22.0(SPSS Inc., USA). Count data were expressed as count (percentage), and the Chi-square test was used for comparison between groups; normally distributed continuous data were expressed as mean ± standard deviation (x ± s), and comparisons between two groups were determined by t-test; skewed distributed continuous data were described as median (25th–75th quantiles), and comparisons between two groups were made by Wilcoxon rank sum test; the survival curves of the two groups were estimated by the Kaplan-Meier method, and the log-rank sum test was used to compare the differences in OS as well as TTP time between the different groups; Univariate and multivariate Cox’s proportional hazards regression analyses were used to determine prognostic factors of OS, and the multivariate Cox’ s proportional hazards regression was performed using forward stepwise (conditional LR) method. p-value <0.05 was considered significant.
Results
Baseline indexes and tumor characteristics of patients in both groups
As for baseline characteristics, no difference was observed between cTACE+DEB-TACE and cTACE groups either regarding to age (p=0.100), gender (p=0.150), BMI (p=0.236) , cause of cirrhosis (p=0.187,0.139), maximum diameter of lesion (p=0.960), number of lesions (p= 0.893), PVTT (p=0.904), number of TACEs (p=0.562),BCLC stage (p=0.901), and combined targeted therapy (p=0.788), as shown in Table II.
Table 2: Baseline clinical indicators for the study population treated with c-TACE+D-TACE and C-TACE
Parameters |
All patients (n=161) |
cTACE+DEB-TACE (n=58) |
cTACE (n=103) |
p-value |
|---|---|---|---|---|
Age |
57.584±10.860 |
55.706±11.077 |
58.641±10.644 |
0.100 |
Gender |
|
|
|
0.150 |
Male |
134 (83.2%) |
45 (77.6%) |
89 (86.4%) |
|
Female |
27 (16.8%) |
13 (22.4%) |
14 (13.6%) |
|
BMI |
23.818±3.317 |
23.399±3.555 |
24.054±3.240 |
0.236 |
Alcoholism |
31 (19.3%) |
8 (13.8%) |
23 (22.3%) |
0.187 |
History of infectious diseases |
|
|
|
0.139 |
Hepatitis B |
113 (70.2%) |
36 (62.1%) |
77 (74.8%) |
____ |
Hepatitis C |
12 (7.5%) |
4 (6.9%) |
8(7.8%) |
-- |
None |
36 (22.4%) |
18 (31.0%) |
18 (17.5%) |
____ |
PVTT |
38 (23.6%) |
14 (24.1%) |
24 (23.3%) |
0.904 |
Number of TACEs |
3.422±1.832 |
3.310±1.287 |
3.485±2.081 |
0.562 |
Average single iodised oil dosage (ml) |
12.609±4.637 |
11.715±4.399 |
13.109±4.711 |
0.069 |
Number of lesions |
|
|
|
0.893 |
1 |
90(55.9%) |
34 (58.6%) |
56 (54.4%) |
|
2 |
27 (16.8%) |
8 (13.8%) |
19 (18.4%) |
|
3 |
8 (5.5%) |
1 (1.7%) |
2(1.9%) |
|
>3 |
41 (25.5%) |
15 (25.9%) |
26 (25.2%) |
|
Maximum diameter of lesion (cm) |
98.706±33.274 |
98.529±31.928 |
98.806±34.163 |
0.960 |
BCLC |
|
|
|
0.901 |
B |
112 (69.6%) |
40 (69.0%) |
72 (69.9%) |
|
C |
49 (30.4%) |
18 (31.0%) |
31(30.1%) |
|
Combined targeted therapy |
18 (11.2%) |
7 (12.1%) |
11 (10.7%) |
0.788 |
Data were presented as mean value ± standard deviation, count (percentage), or median (25th–75th quantiles). Comparison was determined by t-test, Chi-square test, or Wilcoxon rank sum test. p-value < 0.05 was considered significant . DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemoembolization; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer.
Treatment response
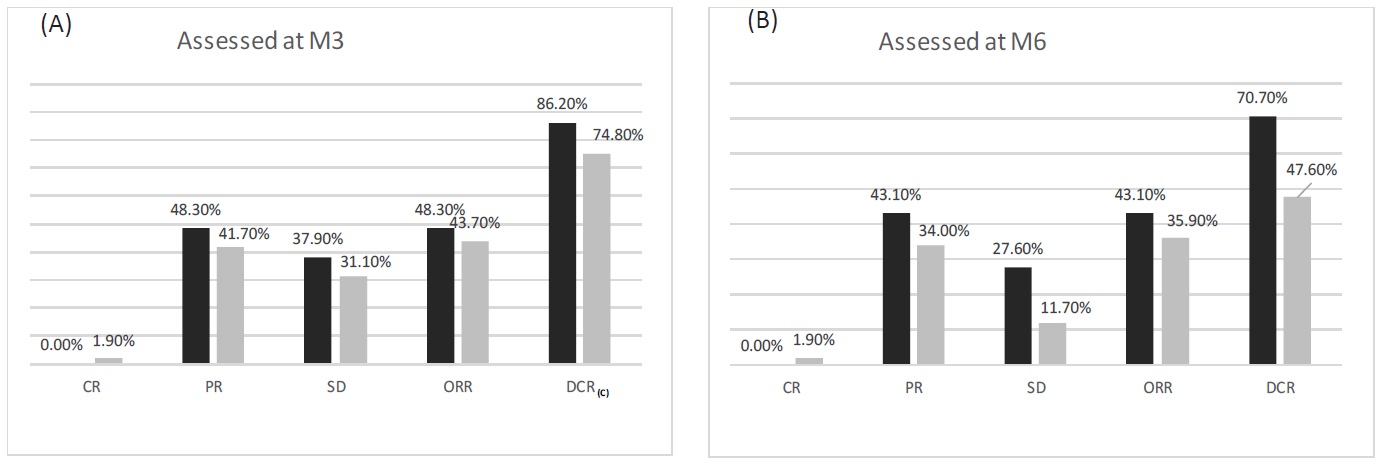
Comparison of treatment response rate between the cTACE+DEB-TACE and cTACE groups was performed using Chisquare test. At M3 after treatment, no difference in CR, PR, SD, ORR, or DCR was observed between the two groups (All p > 0.05) (Figure A). At M6 after treatment, CR,PR,ORR was similar (p > 0.05) but SD (p=0.037 < 0.05) and DCR (p=0.012< 0.05) were higher in the cTACE+DEB-TACE group compared with the cTACE group (Figure B).These implied that cTACE+DEB-TACE resulted in better treatment response in large or giant HCC patients compared with cTACE.
Comparison of TTP and OS between cTACE+DEB-TACE group and cTACE group
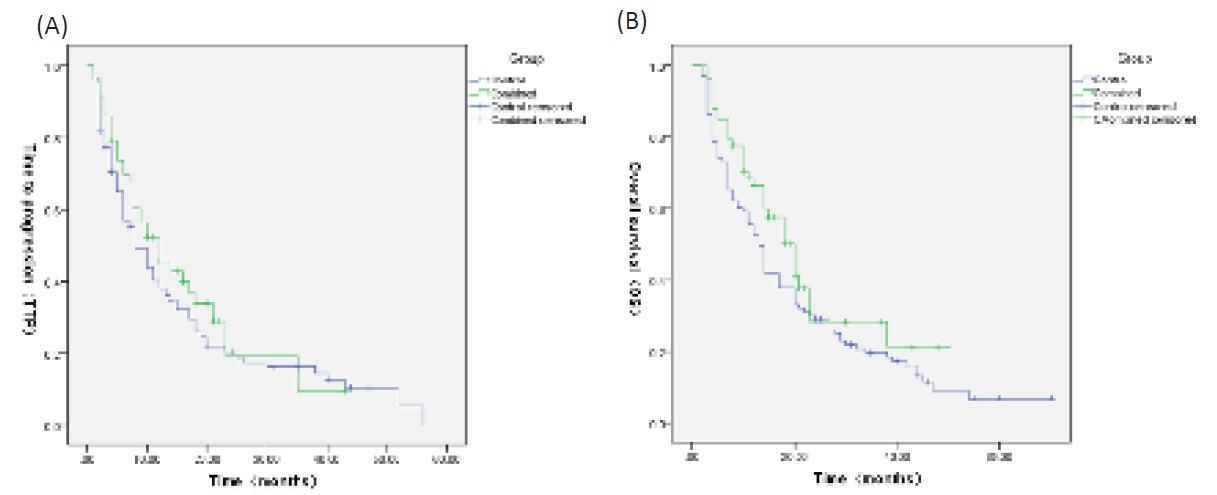
Kaplan-Meier method was used to assess TTP and OS of HCC patients and difference between the cTACE+DEB-TACE and cTACE groups was determined by log-rank test. TTP was similar in the cTACE+DEB-TACE group (median TTP: 16.080 months, 95%CI: 11.636-20.523 months) compared with the cTACE group (median TTP: 15.433 months, 95% CI: 11.931-18.936 months) (p=0.251) (Figure 2A). OS was also similar in the cTACE+DEBTACE group (median OS: 22.909 months; 95% CI: 17.642-28.176 months) compared with the cTACE group (median OS: 20.156 months, 95% CI: 16.317-23.995 months) (p=0.092) (Figure 2B).
Factors affecting OS
Univariate Cox’s proportional hazards regression displayed that BCLC B stage (p = 0.000) and combined targeted therapy (p=0.032)was associated with better OS, whereas PVTT (p= 0.000), number of lesions (n)≥3 (p= 0.002), Largest lesion diameter (cm) >10(p= 0.000), Iodine oil dosage > cutoff value (p= 0.002) were correlated with worse OS. In addition, multivariate Cox’s regression with forward stepwise illustrated that number of lesions (n)≥3 (p=0.027), largest lesion diameter (cm) >10 cm (p=0.005), BCLC C stage (p=0.000) independently predicted shorter OS in HCC patients, as shown in Table III.
Table 3: Univariate and multivariate COX regression model analysis of factors affecting OS
|
|
Univariate Cox's regression |
Multivariate Cox's regression |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
pvalue |
HR |
95% CI |
|
pvalue |
HR |
95% CI |
||
parameters |
|
|
Lower |
Higher |
|
|
|
Lower |
Higher |
Age≥60years |
0.249 |
0.808 |
0.562 |
1.161 |
|
|
|
|
|
Male |
0.483 |
0.834 |
0.502 |
1.386 |
|
|
|
|
|
BMI |
0.487 |
1.135 |
0.794 |
1.624 |
|
|
|
|
|
PVTT |
0.000 |
3.328 |
2.155 |
5.138 |
|
|
|
|
|
Number of lesions (n) |
0.002 |
1.864 |
1.251 |
2.778 |
|
0.027 |
1.582 |
1.055 |
2.375 |
Largest lesion diameter (cm) |
0.000 |
1.991 |
1.386 |
2.861 |
|
0.005 |
1.710 |
1.181 |
2.477 |
BCLC B vs C |
0.000 |
0.273 |
0.181 |
0.412 |
|
0.000 |
0.307 |
0.202 |
0.466 |
AFP >400ng/ml |
0.150 |
1.305 |
0.908 |
1.875 |
|
|
|
|
|
Combined targeted therapy |
0.032 |
1.840 |
1.053 |
3.217 |
|
|
|
|
|
Iodine oil dosage (ml) |
0.002 |
1.777 |
1.241 |
2.544 |
|
|
|
|
|
Factors affecting OS were determined by univariate and multivariate Cox’s proportional hazards regression model analyses, and the multivariate Cox’s proportional hazards regression was performed with forward stepwise (conditional LR) method. p-value < 0.05 was considered significant. OS, overall survival; HR, hazard ratio; CI, confidence interval; PVTT, portal vein tumor thrombus; BCLC, Barcelona Clinic Liver Cancer.
Comparison of laboratory indexes between the two groups
No difference in ALT, AST, PT, TBIL was observed between the cTACE+DEB-TACE and cTACE groups at M0, W1, or M1 (All p > 0.05), whereas at W1,M1 ,ALB (p =0.001,0.035 < 0.05) was lower in the cTACE+DEB-TACE group compared with the cTACE group (Table V).
Table 4: Comparison of laboratory parameters between the cTACE+DEB-TACE and cTACE groups before and after treatment.
Parameters |
Time | cTACE+DEB-TACE (n=58) |
cTACE (n=103) |
p-value |
|---|---|---|---|---|
ALB (g/L) |
M0 |
39.051±4.827 |
40.057±4.715 |
0.222 |
|
W1 |
32.874±3.631 |
35.532±3.748 |
0.001 |
|
M1 |
37.319±4.768 |
38.974±5.149 |
0.035 |
TBIL (umol/L) |
M0 |
21.208±9.875 |
19.649±9.431 |
0.248 |
|
W1 |
45.095±27.019 |
37.236±17.278 |
0.210 |
|
M1 |
26.446±32.036 |
26.064±52.982 |
0.260 |
PT(S) |
M0 |
12.359±1.441 |
12.290±1.000 |
0.425 |
|
M1 |
12.311±1.108 |
12.533±1.068 |
0.143 |
ALT (U/L) |
M0 |
43.194±19.096 |
47.740 ±18.673 |
0.122 |
|
W1 |
128.000(57.000-270.000) |
125.000(68.250-249.500) |
0.779 |
|
M1 |
48.144±35.315 |
47.627 ±39.359 |
0.743 |
AST (U/L) |
M0 |
56.059±29.110 |
56.414±25.219 |
0.572 |
|
W1 |
113.500(55.750-207.500) |
110.000(57.000-198.250) |
0.801 |
|
M1 |
72.593±83.701 |
82.911±104.129 |
0.890 |
Data were presented as mean value ± standard deviation. Comparison was determined by t-test. p-value < 0.05 was considered significant (in bold). DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemoembolization; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin;prothrombin time.
Table 5: Changes in AFP within the cTACE+DEB-TACE and cTACE groups
Parameters |
cTACE+DEB-TACE (n=58) |
cTACE (n=103) |
p-value |
|---|---|---|---|
AFP (ng/ml) |
54.960 (13.330- 1069.200) |
293.700 (10.385-8456.500) |
0.204 |
Follow-up cut-off AFP level |
101.245(12.130-1201.250) |
261.300(9.350-5831.000) |
0.255 |
AFP positivity rate(%) |
33(56.90%) |
68(66.0%) |
0.250 |
AFP response rate(%) |
17(51.5%) |
57(83.8%) |
0.001 |
Data were presented as count (percentage) and median (25th–75th quantiles)。Comparison was determined by Chi-square test or Wilcoxon rank sum test. p-value < 0.05 was considered significant . DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemoembolization.
Adverse effects
Regarding the occurrence of adverse reactions in the two groups, the incidence of inflammatory response (p=0.004) and moderate pain (p=0.000) were lower in the cTACE group compared with the cTACE+DEB-TACE group, whereas no difference in occurrence of vomiting (p=0.889), fever (p=0.424) was observed between the two groups, as shown in Table VI.
Table 6: Occurrence of adverse reactions in the two groups after TACE
Parameters |
cTACE+DEB-TACE (n=58) |
cTACE (n=103) |
p-value |
|---|---|---|---|
Vomiting |
56 (96.6%) |
99 (96.1%) |
0.889 |
Inflammatory response |
11(19.0%) |
5(4.9%) |
0.004 |
Fever |
15(25.9%) |
21 (20.4%) |
0.424 |
Moderate pain |
39 (67.2%) |
29 (28.2%) |
0.000 |
Data were presented as count (percentage). Comparison was determined by Chi-square test or Wilcoxon rank sum test. p-value < 0.05 was considered significant . DEB-TACE, drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemoembolization.
Length of hospitalization and Treatment interval
The number of days of hospitalization in the cTACE+DEBTACE and cTACE groups was 6.911±3.891 vs. 7.441±3.117, respectively, p=0.117; the total number of on-demand treatments in six months was 2.626±1.029 vs. 2.409±0.830, p=0.256; the time interval between the first TACE and supplemental TACE was 55.000 (47.000 - 80.000) vs. 53.000 (46.000 - 62.000) days, p=0.014, as shown in Table VII.
Table 7: Hospitalisation and consumption in both groups
Parameters |
cTACE+DEB-TACE (n=58) |
C-TACE (n=103) |
p-value |
|---|---|---|---|
Number of days of hospitalisation (days) |
6.911±3.891 |
7.441±3.117 |
0.117 |
Number of treatments in six months |
2.626±1.029 |
2.409±0.830 |
0.256 |
Time interval between first TACE and supplemental TACE (days) |
55.000 (47.000 - 80.000) |
53.000 (46.000 - 62.000) |
0.014 |
Data were presented as mean ± standard deviation and median (25th–75th quantiles). Comparison was determined by t-test or Wilcoxon rank sum test p-value < 0.05 was considered significant . DEB-TACE,drug-eluting bead transarterial chemoembolization; cTACE, conventional transarterial chemoembolization.
Discussion
Our results showed (1) no significant difference in TTP and OS in large or giant HCC patients treated with cTACE in combination with DEB-TACE compared to cTACE, but cTACE+DEB-TACE yielded better treatment response compared with cTACE. (2) Compared with cTACE+DEB-TACE,cTACE resulted in decreased level of liver function injury at W1,M1 as well as lower incidence of adverse events after TACE treatments. (3)AFP response rate was higher in the cTACE group compared with cTACE+DEB-TACE group. (4) The treatment interval was longer in the cTACE+DEBTACE group compared to the cTACE group.
Many attempts have been made by clinicians to treat large or giant HCC. Therapeutic approaches, such as surgical resection, Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), and ablation are increasingly investigated for large or giant HCC management, whereas their treatment outcomes are limited by extensive future liver remnant hypertrophy, high morbidity, complicated tumor location as well as severe complications [13]. TACE is the first-line treatment for intermediate to advanced hepatocellular carcinoma. Accumulating studies have shown the advantages of DEB-TACE over cTACE in certain aspects [14-17]. Thus, we suspected that for large or giant HCC patients, cTACE+DEB-TACE might possess more promising treatment outcomes, and compared the treatment response, survival profiles, as well as safety profiles between cTACE+DEB-TACE and cTACE in HCC patients.
DEB-TACE injects drug-loaded microspheres and loaded chemotherapeutic agents into the blood supply arteries of the tumor. The microspheres slowly release the loaded chemotherapeutic agents in a sustained manner, resulting in increased drug concentrations in the tumor and relatively low drug concentrations in normal liver tissue and throughout the body. In the studies of R Golfieri [18], Yang Jun Kang [19], and Jinpeng Li [20], there was no significant difference between DEB-TACE and cTACE in terms of 1-year and 2-year survival, PFS, OS, ORR, and DCR, what did differ was that the DEB-TACE group improved the short-term outcome of HCC, had less postoperative adverse effects milder, and better tolerated by patients. Similarly ,it has also been shown [21-23] that there was no difference between cTACE and D-TACE in terms of OS. In our study, there was no statistical difference between the cTACE+DEB-TACE group and the cTACE group in mTTP, mOS, M3 ORR, M3 DCR and M6 ORR, but the M6 DCR of the cTACE+DEB-TACE group was higher than that of the cTACE group 70.7% vs. 47.60%, p=0.012<0.05, which was basically consistent with the previous study. The DEB used in the cTACE+DEB-TACE group was a permanent embolic material, which had a stronger ability to occlude the tumor blood supply artery than the absorbable gelatin sponge and would not be metabolized and absorbed in the short term, and the microspheres had a better drug loading capacity and stability. Compared with the iodine oil used in cTACE, the drug was concentrated on the target tumor and more effectively killed cancer cells and induced tumor necrosis, which might be the reason why the M6 DCR of the cTACE+DEB-TACE group was higher than that of the cTACE group.
Iodinated oil chemotherapeutic drug emulsion is in liquid form, so it is easier to embolize the end vessels of the tumor and achieve the end embolization of the tumor vessels, and the satellite foci can also achieve embolization. A sufficient amount of iodinated oil can also enter the portal vein to achieve the double embolization effect. The dose of iodized oil is controlled within 20 ml, which can achieve the purpose of effective embolization and control the complications effectively. However, for large lesion HCC with diameter >5 cm, the efficacy is limited. After cTACE iodinated oil chemotherapeutic drug emulsion embolization, gelatin sponge particles embolization is applied. Gelatin sponge particles are large in size and irregular in shape, easily washed by blood flow, and it is absorbable material. The vascular recanalization rate is high, the intimal damage is obvious, and the absence of iodinated oil chemotherapeutic drug emulsion in the tumor vessel portal is obvious. The drug-loaded microspheres are regular spheres [24], which are smaller in particle size compared with gelatin sponges, embolizing tumor vessels more densely, with less damage to the intima, and the permanent embolic material will not be absorbed, while the chemotherapeutic drug is released slowly, effectively reducing many drawbacks such as the embolization of large lesions with simple iodinated oil chemotherapy emulsion being washed away by blood flow, the absence of iodinated oil in the tumor vascular gate, and low local drug concentration.
On the adverse effects of TACE for HCC, a series of studies [14,16,18] have shown that DEB-TACE is milder in terms of damage to liver function, postoperative abdominal pain, and nausea and vomiting due to chemotherapy drugs. Inconsistent with previous studies, our study observed that hepatic injury was less and the incidence of adverse events was lower in the cTACE group compared with the cTACE + DEB-TACE group in large and giant HCC patients. For example, the number of cases requiring opioid analgesics and inflammatory response was higher in the cTACE + DEB-TACE group than in the cTACE group, and the ALB level was lower in the cTACE + DEB-TACE group than in the cTACE group at W1,M1after surgery, but the reactions were mostly transient and could be improved with conventional analgesic and albumin supplementation treatment. Probably due to differences in the type of drug-loaded microspheres and the level of super selected vessels performed by the operator. Additionally, the number of DEB-TACE required was less than the number of cTACE throughout the treatment [25]. In our study, the mean number of treatments required within six months in both groups was 2.626 ± 1.029 (n) vs. 2.409 ± 0.830 (n), p=0.256; the time interval (days) between first TACE and supplemental TACE was longer in the cTACE + DEB-TACE group than in the cTACE group, 55.000 (47.000 - 80.000)vs. 53.000 (46.000 - 62.000), p=0.014.
Limitations
This study is a single-center clinical retrospective study with a small sample size, and most of the included patients are HCC patients in northeast China, so there is a certain degree of selection bias; when performing the efficacy evaluation, the relevant tumor index monitoring failed to detect abnormal prothrombin, plasma free microRNA or serum methemoglobin heterogeneity except for AFP, which lacks a certain degree of persuasiveness; according to the DEB-TACE standard technical operation recommendation, microspheres of appropriate particle size should be selected according to tumor size and blood supply conditions [26], as shown in Table VIII, 100-300 μm drug-loaded microspheres were selected for all treatments in this study, and evaluation of drug-loaded microspheres of other diameters was missing; therefore, further validation in future large sample and multicenter prospective randomized controlled trials is needed.
Table 8: Selection of particle size of DEB
particle size of microspheres |
patient Choice |
|---|---|
70-150μm |
(Evidence of quality: II-2; Level of recommendation: A) |
100-300μm |
(Evidence of quality: II-1; Level of recommendation: A) |
300-500μm |
a. >7cm, rich blood supply, primary hepatocellular carcinoma |
DEB, Drug eluting bead.
Conclusion
In conclusion, compared to cTACE, cTACE combined with DEB-TACE for large or giant HCC presented a superior treatment response profile, and adverse effects were tolerated in both groups, with longer treatment intervals in the combined group.
Declarations
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no competing interests.
Institutional review board statement: The study was reviewed and approved by the Medical Ethics Review Committee of Harbin Medical University Cancer Hospital.
Author contributions: Wang YF and Zeng J drafted the manuscript and analyzed all the data; He DF performed the surgeries and acquired data; Wang HW , Hou YW, Wu J and Lu JR assisted in all surgeries and date collection; He DF designed and supervised the study, analyzed all the data, and revised the manuscript.
Ethics approval: The study was reviewed and approved by the Medical Ethics Review Committee of Harbin Medical University Cancer Hospital.
Consent to participate: Written informed consent was obtained from the parents.
Consent to publish: The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 3a-3g.
Data Availability: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- Sung H, Ferlay J, et al. “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.” CA: A cancer journal for clinicians. 2021; 71: 209-249.
- Zhang Z, Li H, et al. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety. BMC Cancer. 2019; 19: 1162.
- Huang J, Huang W, et al. Drug-Eluting Bead Transarterial Chemoembolization Combined with FOLFOX-Based Hepatic Arterial Infusion Chemotherapy for Large or Huge Hepatocellular Carcinoma.” Journal of hepatocellular carcinoma. 2021; 8: 1445- 1458.
- Huang Y, Wu J, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006; 23: 129-35.
- Song MJ, Park, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011; 23: 521-7.
- Xue T, Le F, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015; 32: 64.
- Gomes AS, Monteleone PA, et al. Comparison of Triple-Drug Transcatheter Arterial Chemoembolization (TACE) With SingleDrug TACE Using Doxorubicin-Eluting Beads: Long-Term Survival in 313 Patients. AJR Am J Roentgenol. 2017; 209: 722-732.
- Liu Y, Huang W, et al. Efficacy and Safety of CalliSpheres(R) DrugEluting Beads Transarterial Chemoembolization in Barcelona Clinic Liver Cancer Stage C Patients. Oncol Res. 2019; 27: 565– 73.
- Zhou Y. Drug-eluting bead transarterial chemoembolization is efficient and well-tolerated in treating elderly Chinese hepatocellular carcinoma patients.Int J Clin Exp Pathol. 2018; 11: 4867- 4878.
- Zhang X, Lin, et al. “An investigation of efficacy, safety, and prognostic factors of drug-eluting beads-transarterial chemoembolization operation with CalliSpheres(®) Microspheres in treating Chinese hepatocellular carcinoma patients.” Journal of clinical laboratory analysis. 2019; 33: e22975.
- Yoshimitsu, K. “Transarterial chemoembolization using iodized oil for unresectable hepatocellular carcinoma: perspective from multistep hepatocarcinogenesis.” Hepatic medicine: evidence and research. 2014; 6: 89-94.
- Wakayama K, Kamiyama T, et al. Huge Hepatocellular Carcinoma Greater Than 10 Cm in Diameter Worsens Prognosis by Causing Distant Recurrence After Curative Resection. J Surg Oncol. 2017; 115: 324–9.
- Duan X, Liu J, Han X, Ren J, Li H, et al. Comparison of Treatment Response, Survival Profiles, as Well as Safety Profiles Between CalliSpheres(®) Microsphere Transarterial Chemoembolization and Conventional Transarterial Chemoembolization in Huge Hepatocellular Carcinoma. Frontiers in oncology. 2021; 11: 793581.
- Arabi, M, Ben Mousa A, et al. “Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma.” Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. Saudi J Gastroenterol. 2015; 21: 175–80.
- Ou M, Liu Y, Chuang M, Lin C, Huang L, Lin C. Time-to-progression following conventional compared with drug-eluting-bead transcatheter arterial chemoembolisation in patients with large hepatocellular carcinoma.Clin Radiol. 2019; 74: 295-300.
- Wu B, Zhou J, et al. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018; 16: 69.
- Zhang X, Zhou J, Zhu DD, Huang J, Sun JH, et al. CalliSpheres(R) Drug-Eluting Beads (DEB) Transarterial Chemoembolization (TACE) Is Equally Efficient and Safe in Liver Cancer Patients With Different Times of Previous Conventional TACE Treatments: A Result From CTILC Study. Clin Transl Oncol. 2019; 21: 167–77.
- Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, et al. Randomised Controlled Trial of Doxorubicin-Eluting Beads vs Conventional Chemoembolisation for Hepatocellular Carcinoma. Br J Cancer. 2014; 111: 255–64.
- Kang YJ, Lee BC, et al. Conventional Versus Small Doxorubicineluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2020; 43: 55-64.
- Li J, Wang N, et al. Short-term efficacy and safety of callispheres drug-loaded microsphere embolization in primary hepatocellular carcinoma. J Cancer Res Ther. 2021; 17: 733-739.
- Liu Y, Lin C, et al. Five-year outcome of conventional and drugeluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma.” BMC gastroenterology. 2018; 18: 124.
- Li H. Wu F, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety. Medicine (Baltimore). 2019; 98: e15314.
- Shimose S, Iwamoto H, et al. Increased Arterio-Portal Shunt Formation after Drug-Eluting Beads TACE for Hepatocellular Carcinoma.” Oncology. 2020; 98: 558-565.
- Melchiorre F, Patella F, et al. “DEB-TACE: a standard review.” Future oncology (London, England). 2018; 14: 2969-2984.
- Kloeckner R, A. Weinmann, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015; 15: 465.
- Shao G, Zou Y, et al. “Chinese expert consensus on technical recommendations for the standard operation of drug-eluting beads for transvascular embolization.” Annals of translational medicine. 2021; 9: 714.