Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Is it possible to withdrawal PPI’s therapy in GERD patients? A prospective study on 216 patients using HYCHSA
F Di Mario1*; M Franceschi2; C Miraglia1; M Russo1; L Franzoni1; K Rodriguez-Castro2; P Crafa1
1Department of Medicine and Surgery, University of Parma, Parma, Italy.
2Endoscopy Unit, Department of Medicine, ULSS7 Pedemontana, Hospital Alto Vicentino, Santorso (VI), Italy.
*Corresponding Author : Francesco Di Mario
Department of Medicine and Surgery, University of
Parma, Via Gramsci, n. 14, 43126, Parma, Italy.
Email: francesco.dimario@unipr.it
Received : Feb 14, 2022
Accepted : Mar 18, 2022
Published : Mar 24, 2022
Archived : www.jjgastro.com
Copyright : © Mario FD (2022).
Abstract
Background and study aim: Therapy of GERD involves acid suppression by anti-acid or Proton Pump Inhibitors; this schedule can fail to relief symptoms and prevent early relapse in nearly 30% of cases.
We evaluate the effect of a medical device, based on an oral combination of hyaluronic acid, chondroitin sulfate and aluminum (HYCHSA) in patients whit GERD, in comparison whit PPI treatment.
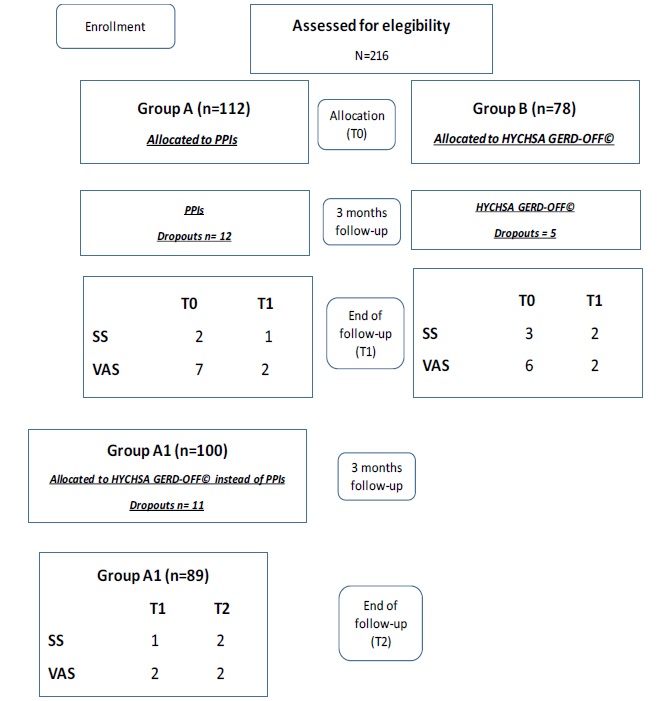
Patients and methods: We selected 216 patients (118 F, mean age 48 yrs., range 22-88), with Los Angeles Grade A esophagitis, typical symptoms and previous history of early relapse. Patients were divided in Group A (112 pts) undergoing PPI full-dose and Group B (78 pts) treated whit a melt-in-mouth medical device (HYCHSA, 1100 mg: GERD-off©) three times a day for 3 months. Clinical outcome assessed by Visual analogical scale (VAS) and Symptomatic Score (S.S.). VAS and S.S. collected at baseline (T0) and after three months (T1). In a second schedule, 100 pts from Group A (Group A1) started a three months therapy taking HYCHSA after stopping PPIs.
Results: In Group A S.S. was 2 (T0), and 1 at T1; VAS was 7 (T0) and 2 at T1. In Group B treated whit HYCHSA, S.S. was 3 (T0) and 2 at T1; VAS was 6 (T0) and 2 at T1. In Group A1 after 3 months taking HYCHSA, S.S. was 2 and VAS 2.
Conclusion: Oral association of HYCHSA may represent a real alternative to PPI therapy in GERD pts, by both improving symptoms and prevent early relapse.
Keywords: GERD; HYCHSA; relapse; esophagitis.
Citation: F. Di Mario, Franceschi M, Miraglia C, Russo M, Franzoni L, et al. Is it possible to withdrawal PPI’s therapy in GERD? A prospective study on 216 patients using HYCHSA. Japanese J Gastroenterol Res. 2022; 2(5): 1071.
Introduction
Proton Pump Inhibitor (PPI) therapy is claimed to be of reference for medical treatment of Gastroesophaegeal Reflux Disease (GERD) [1,2].
The healing of esophagitis, in fact, is reported in more than 80% of patients [3], although the control of GERD symptoms is less satisfactory [4].
In particular, the so-called Non-Erosive Reflux Disease (NERD) subjects show no more than 60% of symptomatic response and needed at least one month of PPI full-dose therapy to achieve the result [2,5-7]. The reason for these unsatisfactory results included different factor like the role of bile and pepsin in refluxate as well as weekly acid reflux, motility alterations of both esophagus and stomach and finally disruption of esophageal mucosal barrier [8-10]. In some subjects, hypersensitivity to acid or other reflux constituents or so-called “irritable esophagus” are associated with failure of therapy [11-13]. Beside PPIs, other drugs are currently used for GERD therapy, including antiacid, pro-kinetics, alginates alone or in association, but usually when needed and not intended as causal therapy. The results of the studies based on the use of such compounds are controversial and of difficult comparison each other [14,15]. In the last years, new medical devices based on association of compounds currently used in the past to protect esophageal, gastric and duodenal mucosal integrity against aggressive factors and to contribute to healing of erosion and ulcers, have been proposed for GERD therapy. They include hyaluronic acid involved in the turn-over of epithelial cells, healing of ulcers and ri-epithelization of upper Gastro-intestinal (G.I.) mucosa [16-20], and chondroitin sulfate, a glycosaminoglycan, secreted in the upper part of stomach, able to inhibit pepsin induced damages of both gastric and duodenal mucosa [21-24]. Some studies reported that by using this two compounds as add-on therapy to PPIs, it’s possible to improve symptoms in NERD patients [25,26]. A melt-in mouth, tablet using the two compounds plus aluminum (HYCHSA: 1100 mg, GERD-OFF© SOFAR S.p.A., Trezzano Rosa, Italy) has been administered in 2019 to a group of subjects with poor response to alginates and/or PPIs, inducing a satisfactory symptoms relief in symptomatic patients [27].
A possible explanation for such clinical results is represented by an experimental model of esophageal mucosa evaluating its permeability in presence of different formulations containing hyaluronic acid and chondroitin sulfate [28]. The study confirms the ability of such products to adhere and create a stable protective pH for at least two hours of their homogeneous distribution on the epithelium surface, against caffeine penetration at different pH conditions.
Aim
To evaluate the effect of a medical device based on a melting mouth combination of hyaluronic acid chondroitin sulfate plus aluminum (HYCHSA: 1100 mg), in patients affected by GERD in comparison with PPIs treatment.
Material and methods
Inclusion criteria: We selected 218 consecutive patients (118 F, mean age 48 yrs, range 22-88 yrs) with endoscopically proved diagnosis of esophagitis, Grade A, according with Los Angeles classification [29], showing typical symptoms (regurgitation and/or heartburn) with previous relapse after withdrawal of full dose PPIs in the last six months. The sample was collected in a unique gastroenterological center located in North-east of Italy. This is a prospective, open label, not randomized monocenter study.
Exclusion criteria: All patients were Helicobacter pylori (Hp) negative, tested by at least two tests (UBT, HPsA, histology of gastric mucosa) and negative for history of digestive neoplasia, previous peptic ulcer history, chronic hepatic and/or renal diseases.
Study design
After signing the informed consent patients underwent baseline visit, with collection of demographics, information about dietary and life-style habits, as well as presence and severity of GERD symptoms.
Patients were divided in two groups: Group A (112 patients, 59 F, mean age 49, range 26-86 yrs) undergoing three months of PPI full-dose treatment and Group B (78 patients, 41 F, mean age 46 yrs, range 28-88 yrs) treated with tablets containing: HYCHSA, 1100 mg, GERD-OFF©, three times a day (after breakfast, after lunch, at bedtime) for 3 months. Table 1 summarizes epidemiology and clinical data of the two groups.
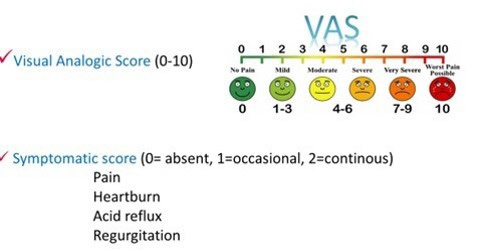
Clinical outcome of therapy was assessed by means of tools: visual analogic scale (VAS ranging from 0-10 where 10 represents the worst bearable pain and symptomatic score (S.S.) which attributes three different scores to each of the four considered symptoms (heartburn, regurgitation, acid reflux, retrosternal pain). Severity was graduated as follows: 0= absence of symptoms, 1= occasional, 2= continuous, with global score ranging from 0 to 8 altogether. Both VAS and S.S. were collected in a semi structured way at baseline (T0) and after the three months period of follow-up on therapy (T1) (Figure 1).
Table 1: Epidemiological data
|
Group A |
Group B |
p |
Total number |
112 |
78 |
|
Female |
59 (53%) |
41 (52%) |
ns |
Male |
53 (47%) |
37 (48%) |
ns |
Mean age |
49 |
46 |
ns |
Range |
26-86 |
28-88 |
ns |
Smoking habit |
21 (19%) |
16 (21%) |
ns |
After the first three month of the study, 100 patients (52 F, mean age 48, range 26-86 yrs.) from group A (Group A1) started a three-month therapy by using in mouth tablets: HYCHSA, 1100 mg, three times a day after stopping PPIs
Figure 2 summarized the flow-chart of the study with subjects’ distribution according with the study design
Statistical analysis
Continuous variables were reported as mean, standard Deviation (SD), range; 95% confidence interval were provided for mean when applicable. Changes from baseline for VAS and S.S. were evaluated by student test for pair data. All statistical analysis were performed using SPSS 28.0 statistics software.
Ethics: Study was conducted according to Helsinki declaration. All patients signed the informed consent to participate in the study.
Results
In Group A, S.S. was 2 (T0), after three months was 1 (T1); VAS was 7 (t0) and 2 after three months of PPI therapy.
In Group B treated with HYCHSA, S.S. was 3 (T0), after three months was 2 (T1); VAS was 6 (t0) and 2 after three months of PPI therapy.
In Group A1 treated with HYCHSA after withdrawal of PPIs, S.S. was 1 (T1), after three months was 2 (T2); VAS was 2 (T1) and 2 (T2) after three months of follow-up.
During the follow-up we experiences overall 28 dropouts (12 in Group A, 5 in Group B, 11 in Group A1).
Fig 2 summarizes the overall results.
Discussion
PPIs represent the therapeutical reference for medical treatment of GERD [1].
To maintain or not a continuous administration of PPIs in GERD patients is matter of debate, for different reasons.
First of all, a large amount of subjects suffering of reflux shows a relief of symptoms after 4-8 weeks taking PPIs and maintains the remission for long time. As a second point, the compliance of patients assuming for long time such drugs in absence of noising troubles are low, and in current life the asymptomatic subjects spontaneously withdrawal PPIs [7,30].
Additionally, PPIs use is claimed to impact with intestinal microbiota when the assumption is prolonged for long times [31,32]. However, a group of GERD patients (nearly 30%) present early relapse of symptoms after withdrawal of PPIs [4]. In the past, some products, often registered as medical devices, where introduced in the schedule of medical therapy of GERD, aiming to assure comparable quality of life to patients instead of use more powerful drugs like PPIs [14,15].
In our study, the use of a medical device as HYCHSA, 1100 mg, GERD-OFF© assumed as in a melted mouth tablet, in a group of GERD patients showing typical symptoms, compared with a PPIs taking sample, showed comparable results both as regard symptoms and global patients’ satisfaction, lasting a three months period of administration. Between possible mechanisms to explain this clinically significant result, we underline the protection of both pharyngeal and upper esophageal mucosa against acid and weekly acid refluxes, covering day and night, when HYCHSA is administered three times a day. Additionally, the melt in mouth formulation, requiring mastication, suckling and grinding lead to an increase of stimuli to salivation and swallowing, each -in turn- increase the properties of HYCHSA adding bicarbonates, in the adhesion of the product to the pharyngeal-esophageal mucosa. Notably, to better explain these results, PPIs are certainly able to reduce acid events, but cannot prevent the contact between epithelium of esophagus and weekly acid refluxes [8]. On the contrary, HYCHSA contributes to build a mechanical barrier directly over the esophageal mucosa, offering a more complete protection against the inappropriate contacts between esophageal mucosa and potentially damaging represented by both acidic or weekly reflux contents.
One important clinical issue in GERD management consists in the choice for better therapy after withdrawal of PPIs in GERD patients experiencing early relapse of symptoms. To try to contribute to this issue, in the second part of the study we shifted the subjects previously treated with PPIs in a group scheduled to assume a more simple therapy by using a medical device like HYCHSA, 1100 mg, becoming such patients their own controls, in a follow up lasting three months. We decided to administer melting mouth tablet of HYCHSA, 1100 mg, three times a day after breakfast and lunch, to prevent post prandial reflux and to offer protection to esophageal mucosa in those critical times and at bedtime to prevent nocturnal reflux. The results are promising, showing that is possible to withdrawal PPIs in GERD patients whit typical symptoms, showing Los Angeles A esophagitis, inducing reduction in S.S. score and improving the global satisfaction assessed by VAS.
Strengthen of the study are represented by: A) large sample of GERD subjects enrolled, B) homogeneity of severity of GERD (Los Angeles esophagitis, grade A) as well as of symptoms (only typical ones), C) comparison between patients under PPIs and subjects treated with HYCHSA, 1100 mg, D) duration of followup: three months, E) evaluation of early relapse of GERD using every patient as its own control.
Weakness of the study: A) open study lacking a standardized randomization, B) GERD diagnosis and results not supported by gold-standard test i.e. pH impedance, C) lacking inclusion of patients whit atypical symptoms, D) no count pills in order to monitoring compliance, E) no evaluation of quality of life using SF36 or similar questionnaires, but only VAS.
Conclusion
In recent years research provides large amounts of data exploring mechanisms underlying esophageal mucosal defense, however the clinical fall out of this enhancement in knowledge on current practice is very low. In fact, irrespective to the existence of a lot of drugs able to reinforce mucosal defenses, specific trial designed to explore and support this issue are lacking.
This study on a large sample of GERD patients provides a statistically significant support to the approach to therapy in such patients using a medical device like HYCHSA, 1100 mg, GERDOFF©, instead of PPIs, suggesting possibility to withdrawal PPIs in prevention of early relapse.
Authors’ contributions
P. Crafa, F. Di Mario : study design, writing of the manuscript, supervision;
K.Rodriguez-Castro, M. Franceschi: clinical data collection and analysis;
C. Miraglia, L. Franzoni, M. Russo: data collection on analysis of the literature.
All authors critically revised the manuscript, approved the final version, and agree to be accountable for all aspects of the work.
References
- Savarino V, Di Mario F. Scarpignato C. Proton pump inhibitors in GORD An overview of their pharmacology, efficacy and safety. Pharmacol Res. 2009; 59: 135-53
- Tack J, Fass R approaches to endoscopic-negative reflux disease: part of the GERD spectrum or a unique acid-related disorder? Aliment Pharmacol Ther . 2004; 19: 28-34
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P Medical treatments in the short-term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007; 18: CD003244.
- Fass R, Shapiro M, Dekel R, Sewell JSystematic review: protonpump inhibitor failure in gastro-oesophageal reflux disease-- where next? Aliment Pharmacol Ther. 2005; 22: 79-94.
- Katz PO, Scheiman JM, Barkun AN acid-related disease--what are the unmet clinical needs? Aliment Pharmacol Ther. 2006; 23: 9-22.
- Richter JE. How to manage refractory GERD. Nat Clin Pract. Gastroenterol Hepatol. 2007; 4: 658-664.
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009; 58: 295-309.
- Tsoukali E, Sifrim D. The Role of Weakly Acidic Reflux in Proton Pump Inhibitor Failure, Has Dust Settled? J Neurogastroenterol Motil. 2010; 16: 258-64.
- Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease NERD. - acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003; 17: 537-545.
- Emerenziani S, Sifrim D, Habib FI, et al. Presence of gas in the refluxate enhances reflux perception in non-erosive patients with physiological acid exposure of the oesophagus. Gut. 2008; 57: 443-447.
- Zerbib F, Duriez A, Roman S, Capdepont M, Mion F. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut. 2008; 57: 156-160.
- Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on acid suppressive therapy. Am J Gastroenterol. 2008; 103: 1090-1096.
- Knowles CH, Aziz Q Visceral hypersensitivity in non-erosive reflux disease Gut. 2008; 57: 674-83
- Reimer C, Lødrup AB, Smith G, Wilkinson J, P Bytzer P. Randomised clinical trial: alginate Gaviscon Advance. vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor Aliment Pharmacol Ther. 2016; 43: 899-909
- Coyle C, Crawford G, Wilkinson J, Thomas SJ, Bytzer P. Randomised clinical trial: addition of alginate-antacid Gaviscon Double Action. to proton pump inhibitor therapy in patients with breakthrough symptoms. Aliment Pharmacol Ther. 2017; 45: 1524-1533.
- Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M Therapeutic applications of hyaluronan. Mol Biosyst. 2010; 6: 437-43.
- Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan Curr Med Chem. 2009; 16: 1718-45.
- Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration J Oral Pathol Med. 2006; 35: 461-5.
- Kapoor P, Sachdeva S, Sachdeva S Topical hyaluronic Acid in the management of oral ulcers. Indian J. Dermatol. 2011; 56: 300-2.
- Ialenti A, Di Rosa M. Hyaluronic acid modulates acute and chronic inflammation. Agents Actions. 1994; 43: 44-7.
- Lauder RM Chondroitin sulphate: a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med. 2009; 17: 56-62
- Volpi N. Anti-inflammatory activity of chondroitin sulphate: new functions from an old natural macromolecule Inflammopharmacology. 2011; 19: 299-306.
- du Souich P, García AG, Vergés J, Montell E Immunomodulatory and anti-inflammatory effects of chondroitin sulphate J Cell Mol Med . 2009; 13: 1451-63.
- Campo GM, Avenoso A, Campo S, Ferlazzo AM, Calatroni A Chondroitin sulphate: antioxidant properties and beneficial effects Mini Rev Med Chem. 2006; 6: 1311-20.
- Savarino V, Pace F, Scarpignato C, Esoxx Study Group Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017; 45: 631-642.
- Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitinsulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci. 2013; 17: 3272-8
- Boarino V, Raguzzi I, Marocchi M, Merighi A. Symptomatic response to GERDOFF® in patients with gastro-esophageal reflux disease and poor response to alginates: an exploratory, postmarket, open-label study. Turk J Gastroenterol. 2020; 31: 466- 473
- Pellegatta G, Spadaccini M, Lamonaca L, Craviotto V, D’Amico F, Ceriotti L. et al. Evaluation of Human Esophageal Epithelium Permeability in Presence of Different Formulations Containing Hyaluronic Acid and Chondroitin Sulphate. Med Devices Auckl.. 2020; 13: 57-66.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic Assessment of Oesophagitis: Clinical and Functional Correlates and Further Validation of the Los Angeles Classification. Gut. 1999; 45: 172– 180.
- Bredenoord AJ, Smout AJ. Refractory gastrooesophageal reflux disease. Eur J Gastroenterol Hepatol. 2008; 20: 217-2.
- Bruno G, Piera Z, Rocco G, Scalese G, Panetta G, Porowska B, et al. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J Gastroenterol. 2019; 25: 2706-2719.
- Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017; 14: 697-710.