Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Colorectal cancer xenografts regressed by a FliC expressing Tanapoxvirus in an immunologically reconstituted model
Sadia I Kana; Michael L Monaco; S Kohler; R Eversole; Karim Essani*
Laboratory of Virology, Department of Biological Sciences, Western Michigan University, Kalamazoo, MI, 49008, USA.
*Corresponding Author : Karim Essani
Laboratory of Virology, Department of Biological Sciences, Western Michigan University, Kalamazoo, MI, 49008, USA.
Email: karim.essani@wmich.edu
Received : Feb 10, 2022
Accepted : Mar 09, 2022
Published : Mar 14, 2022
Archived : www.jjgastro.com
Copyright : © Essani k (2022).
Abstract
Background: Colorectal cancer (CRC) is the third most common neoplasm in the world and the third leading cause of cancer-related deaths in the USA. Immuno-oncolytic virotherapy has emerged as a potential treatment modality for CRC. We have previously shown that a Tanapoxvirus recombinant armed with the fliC gene, TPV/Δ2L/Δ66R/FliC, resulted in a significant reduction of HCT116 tumors in athymic nude mice. The present study was designed to evaluate the immuno-oncolytic efficiency of this virus in an immuno-competent model.
Methods: CRC tumors were induced by subcutaneous injection of HCT116 cells on both the left and right flanks of Balb/C nude mice. The immune system in these mice was reconstituted by the adoptive transfer of splenocytes from immunologically matched, immuno-competent Balb/C mice.
Results: A single intratumoral injection of the virus resulted in a significant and robust regression of the injected tumors (P<0.01) and an almost complete eradication of 80% of these tumors by the time the experiment was terminated. Interestingly, reduction in non-injected tumors contralateral to the injected ones could also be observed which may suggest the induction of systemic anti-tumor immunity.
Conclusion: These results indicate that the recombinant TPV/Δ2L/ Δ66R/FliC is a potential candidate for the treatment of colorectal cancers in humans and should be explored further for the complete realization of its potential.
Keywords: tanapoxvirus; colorectal cancer; oncolytic virotherapy; flagellin c; xenografts; viroimmunotherapy.
Abbreviations: TPV: Tanapoxvirus; CRC: Colorectal Cancer; OV: Oncolytic Virus; TK: Thymidine Kinase; TLR5: Toll-like Receptor 5; OMK: Owl Monkey Kidney Cells.
Citation: Kana SI, Monaco ML, Kohler S, Eversole R, Essani K. Colorectal cancer xenografts regressed by a FliC expressing Tanapoxvirus in an immunologically reconstituted model. Japanese J Gastroenterol Res. 2022; 2(4): 1068.
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world [1] and the third leading cause of cancer-related deaths in the USA [2,3]. In 2022, an estimated 106,180 new colon and 44,850 new rectal cancer cases will be diagnosed in the USA, with an estimated 52,580 deaths [4]. Even though the mortality and morbidity rates are declining among individuals who are 55 years or older, there is an increasing incidence and death rate from CRC among populations younger than 55 [4]. Despite the current improvements in detection techniques, about a fifth of CRC patients develop tumor metastasis at the time of diagnosis, which results in significantly decreased overall survival [5].
The common treatment methods for CRC incorporates surgical resection, radiation therapy, chemotherapy, targeted therapy, and immunotherapy [6]. A treatment regimen for CRC may include a combination of these therapeutic options. For CRC metastasis, a multimodal treatment approach is indispensable [7]. Even though the longevity and overall quality of patients’ lives have improved due to better detection and treatment modalities, the mortality and morbidity from CRC still are very high. Moreover, the current treatment options for CRC typically are associated with side-effects, sometimes to a severe degree. The five-year survival rate for metastatic CRC still is low [5]. Therefore, a novel, safer, and more effective therapeutic approach against CRC is of urgent necessity.
Oncolytic virotherapy (OV therapy), which uses viruses to destroy cancer cells selectively or preferentially, has emerged as a promising treatment for CRC [8,9]. Besides the direct destruction of cancer cells by cytolysis, these viruses can also stimulate the host immune components for an improved anti-cancer effect [10]. The lysis of cancer cells may release danger associated molecular patterns (DAMPs) and thus alert the immune system of potential dangers [8]. Many oncolytic viruses (OVs) are armed with different immune stimulatory gene(s) to enhance immune stimulation against tumors [11]. Many virus platforms, including adenovirus, vaccinia virus, herpes simplex virus, vesicular stomatitis virus, measles virus, Newcastle disease virus, and Tanapoxvirus, have been evaluated for their oncolytic potentials in numerous cancers.
Among various OV platforms, poxviruses are attractive candidates for OV therapy. These are large, enveloped viruses of the poxviridae family with a linear double-stranded DNA genome [12]. Several inherent properties of poxviruses have earned them the title “oncolytic battleships” [13]. The stability of virus preparation and the ease of high titer virus stock production add to poxviruses’ advantages as OVs [14]. Moreover, they bear a good safety profile, and their genomes can accommodate many transgenes. The replication of poxviruses is restricted to cell cytoplasm; hence, their genomes do not integrate into the host chromosomes (reviewed in [15]. A built-in array of immune-modulatory genes help these viruses evade host immune surveillance [16]. Several licensed or established antiviral agents are available, should the virus infection become severe [17].
The poxvirus used in the present study is the recombinant Tanapoxvirus TPV/Δ2L/Δ66R/FliC, which is a member of the genus Yatapoxvirus. This virus was made deficient for the genes 66R (which encodes thymidine kinase) and 2L (which encodes a glycoprotein with anti-TNF binding activity) and has been armed with the FliC gene that encodes for the protein Flagellin C [9]. Apart from equatorial Africa, where the virus is endemic, the global population is likely to be immunologically naïve to this virus [18,19]. Tanapoxvirus causes a self-limiting febrile illness in humans, and the infection is normally confined to the periphery [20]. The human-to-human transmission of this virus is not known to occur. All these features are highly desirable from the perspective of OV selection.
The expression of the enzyme thymidine kinase (TK), which converts thymidine to thymidine monophosphate, is constitutively high in neoplastic cells, whereas the normal cells have peak TK activity during the S phase of the cell cycle and barely express TK at other times [9]. Thymidine kinase is a requisite for DNA synthesis, and hence, TK gene deficiency makes many viruses, particularly poxviruses preferentially replicate in cancer cells. Indeed, TK gene’s ablation has been associated with increased onco-selectivity of many OVs [9,21-25].
Oncolytic viruses have been armed with different immunestimulatory genes [9,26-30] to harness the immune system’s power against different types of cancer. The protein Flagellin C is the main constituent of Bacterial flagellum [31]. Bacterial flagellin is a cognate ligand for toll-like receptor 5 (TLR5) [32,33]. The flagellin binding to TLR5 activates mitogen-activated protein (MAP) kinases and NF-κB through the MyD88-dependent intracellular signaling cascade, which results in the transcription and secretion of pro-inflammatory cytokines [34-36]. Our previous study has shown that TPV/Δ2L/Δ66R/FliC caused a significant reduction of HCT116 tumors in athymic nude mice [9]. A substantial lymphocytic and macrophage responses to the presence of tumor masses with scattered lymphocytic invasions of the main tumor tissue could be observed. The current study was built on these previous experimental findings and was designed to evaluate the anti-tumor effect of this Tanapoxvirus recombinant in immune-competent models. Here, we hypothesized that TPV/Δ2L/Δ66R/FliC would be able to regress HCT116 tumors in immuno-competent mice.
Materials and methods
Cells, reagents, and viruses
Owl Monkey Kidney (OMK) cells and CRC cell line HCT116 were purchased from the American Type Culture Collection (ATCC product numbers are CRL-1556 and CCL-247 respectively). OMK cells were used for the proliferation and titration of the virus. The cell lines were propagated in complete growth medium consisting of Eagle’s Minimum Essential Medium (EMEM) and Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco/Life Technologies), for OMK and HCT116 cells respectively. Both media were supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Atlanta Biologicals), 2 mM L-glutamine (Sigma-Aldrich) and 50 μg/ml of penicillin and streptomycin. The virus infected cell monolayers were maintained in maintenance medium identical to the growth medium except for the concentration of FBS, which was reduced to 2%. All cells were incubated at 37°C in a 5% CO2 atmosphere. Cell counting and cell viability assays were done with an improved Neubauer hemacytometer using 0.2 % (wt/vol) trypan blue in a normal saline solution. Wild-type TPV (Kenya strain) was originally a gift from Dr. Joseph Esposito (Centers for Disease Control, Atlanta, GA, USA). It was genetically modified in the laboratory of G. McFadden to express the fluorescent reporter enhanced green fluorescent protein (EGFP), but without any further modifications.
density determinations
The HCT116 cells were inoculated into 12-well plates (3 wells per cell line) such a way that one day later the cells were 90% confluent. The cells were then trypsinized, counted and scored for viability by trypan blue exclusion to ensure that the same number of cells were injected into the mice and all mice develop tumors at around the same time.
Virus titration
We performed a plaque assay according to the instructions previously described [37] to determine the number of viable virions present in a sample. Briefly, virus samples were subjected to three rounds of freezing and thawing at −80°C, sonicated for 15 seconds on ice, serially diluted in maintenance medium, and inoculated onto nearly confluent OMK cell monolayers in 6-well plates. The virus was allowed to adsorb at room temperature with gentle rocking for one hour. The inoculum was then removed and each well washed two times with 1 ml of pre-warmed (37°C) maintenance medium. After washing, 2 ml of overlay medium (containing 0.5% methylcellulose in maintenance medium) was added and the infected OMK monolayers incubated for 8 days at 37°C. The overlay medium was then removed, and monolayers were stained (0.1% crystal violet in 37% formaldehyde). Plates were washed with distilled water, dried in the air, and plaques were counted on a light box. Each experiment was independently repeated three times.
Animals
Female athymic inbred Balb/C (homozygous) mice (CAnN. Cg-Foxn1nu/Crl) and regular female inbred Balb/C (heterozygous) mice (BALB/cAnNCrl) were purchased from the Charles River Laboratories at four weeks of age and allowed to acclimate for one week before experimentation. Mice were individually housed in clear polycarbonate cages under a 12-hour light/dark cycle. Food and water were available ad libitum. All animal housing conditions, manipulations and treatments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Western Michigan University (IACUC protocol approval number 19-06-04).
Tumor induction and measurement
Tumors were induced in the athymic mice by subcutaneous injection of 6 X 106 HCT116 cells on the left and right flanks of each mouse. Each injection was followed by an assessment of viability by trypan blue exclusion to ensure that the cells were viable at and after the time of injection. Once visible, tumors were measured using a digital caliper (Pittsburgh, model 6ZBTMCO) along the major axis (length), minor axis (width) and z dimension (height), which were substituted into the volume formula=(length) X (width) X (height) X (π/6). When tumor size surpassed 100 mm3 , the animals were randomly segregated into the vehicle control group, immune-reconstitution control group or virus treatment group.
Virotherapy of HCT116 Xenografts in nude mice
The virus treatment group was composed of five tumor-bearing athymic nude mice. A single virotherapeutic injection of 5 × 106 plaque-forming units (pfu) suspended in 100 μl Dulbecco’s phosphate-buffered saline (DPBS) solution was administered intratumorally once tumor volume exceeded 100 mm3. Both the vehicle and immune-reconstitution control groups consisted of three animals and experienced only a mock virotherapeutic injection (100 μl of vehicle DPBS only). Mouse weights and tumor volumes were measured and recorded at every other day thereafter. Data were collected for a total of 40 days.
Reconstitution of immune system
Thirteen days after virotherapy or mock therapy, the mice in the virus treatment and immune-reconstitution control groups were immune reconstituted by the adoptive transfer of 3 x 106 splenocytes/mouse from the immunologically matched immuno-competent Balb/C mice. At first, the spleens from the immuno-competent mice were harvested and kept in a beaker containing DMEM. The spleens were placed into a cell strainer and mashed with a cell scraper through the cell strainer into a beaker containing DMEM to isolate the splenocytes. The cell strainer was rinsed with DMEM to collect any remaining splenocytes into the beaker. The splenocytes suspended in DMEM were transferred into a 15 ml tube and centrifuged at 1000 RPM for 8 minutes. The supernatant was discarded, and the pellet was re-suspended in DPBS. The splenocytes suspended in DPBS were centrifuged at 1000 RPM for 8 minutes again. Afterward, the supernatant was discarded, and the cells were suspended in DPBS. The cells were then counted and scored for viability by trypan blue exclusion to ensure the desired number of 3 x 106 cells injected into each mouse.
Statistics
The virus treated (virus + immune-reconstitution) group was compared to the immune-reconstitution control and vehicle control groups to assess the therapeutic efficacy of the virus using the Satterthwaite’s method. The analysis was carried out using percent of initial tumor volume as the response variable. The response variable was log-transformed so that the model assumptions were satisfied. Day 0 was not included in the analysis because all subjects had the same value on that day (i.e., Percent of Initial Volume = 100 on Day 0). Virotherapeutic treatment was considered to have produced a significant therapeutic effect if the average tumor volume at a specific time point and within a group was significantly reduced when compared to the control groups. The significance codes used in the study: 0‘***’ 0.001‘**’ 0.01‘*’ 0.05 ‘.’ 0.1 ‘..’ 1.
Results
TPV caused regression of the injected tumors
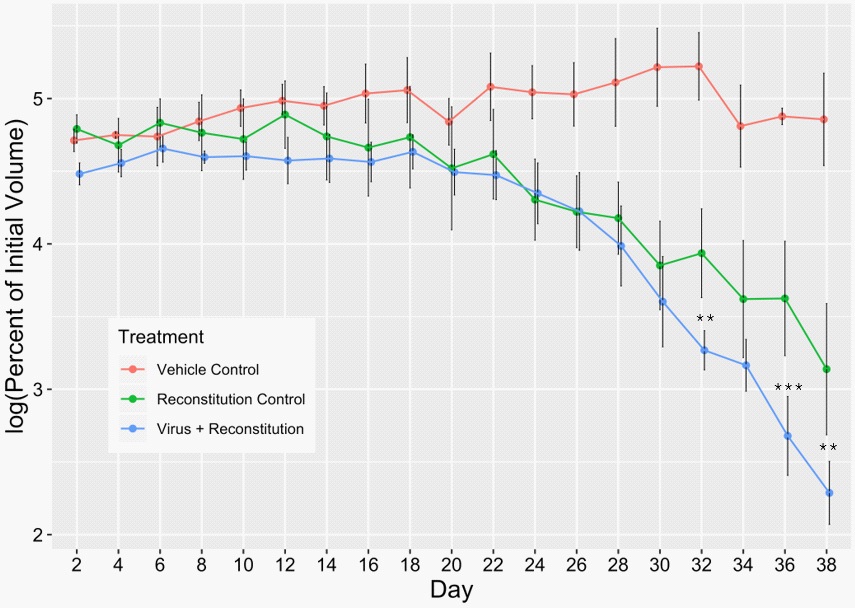
Concentrated stocks of TPV/Δ2L/Δ66R/FliC were produced using previously optimized protocols in OMK cells [9]. Concentrated virus stock showed a titer of 1 × 1010 pfu/ml. This virus stock was then diluted to a dosage of 5 × 106 pfu/ml that was used in the current study. Tumors were then induced by subcutaneous injections of 6 X 106 HCT116 cells on both the left and right flanks of inbred Balb/C nude mice to determine the immuno-oncolytic efficacy of TPV/Δ2L/Δ66R/FliC against hCRC. Upon development of the tumors, mice were randomly distributed into three groups: 1. virus treatment (virus + immunereconstitution) group, 2. immune-reconstitution control group, and 3. vehicle control group. Once one of the two tumors in a mouse surpassed a volume of 100 mm3 , the mouse was subjected to either virotherapy or mock therapy with DPBS, and we marked this time as day 0. The tumor volumes were measured every other day, starting from day 0. On day 14, the mice from groups 1 and 2 were immune-reconstituted by the adoptive transfer of 3 x 106 splenocytes per mouse intraperitoneally (IP). The virotherapeutic results for the injected tumors are presented in (Figure 1). Both the immune-reconstitution control and the virus treatment groups showed a sharp decline in tumor volumes compared to the vehicle control group. The virus treatment group resulted in a better tumor regression compared to both the control groups. When the percent changes in tumor volume from day 24 to day 38 were compared, the virus treatment group demonstrated a significant tumor regression at three time points (day 32, 36 and 38) compared to the immune-reconstitution control group. When compared to the vehicle control group, treatment with TPV/Δ2L/Δ66R/FliC was significantly different at almost every data point. Nearly complete reduction of injected tumors could be observed for 80% of the mice in the virus treatment group prior to the experiment’s termination. These results strongly suggest that the mice were immuno-competent and establish the anti-tumor efficacy of this virus in immuno-competent mice.
TPV caused regression of non-injected contralateral tumors
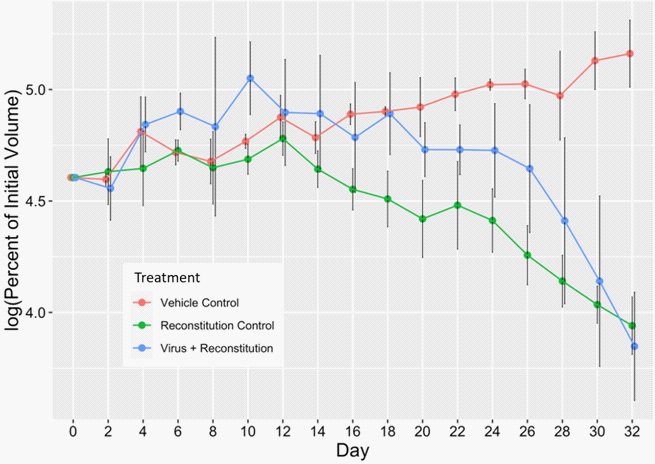
To determine whether the intratumoral virotherapy of primary tumors can cause regression of the non-treated metastatic tumors, we induced and recorded the volume of a second tumor contralateral to the primary injected tumors. Analyses of these non-injected tumor volumes showed that both the immune-reconstitution control and the virus treatment groups had a sharp regression of tumor volumes compared to the vehicle control group. When the percent changes of tumor volume data from day 24 through day 32 were compared, both the groups showed significant tumor regression at all time points compared to the vehicle control group. There was an obvious tumor regression in the virus treated group, and on day 32, the group showed a higher tumor regression than the immune-reconstitution group. However, no significant differences in the tumor regression could be observed between the virus treatment and immune-reconstitution control groups.
Discussion
Here, we present one of the first-ever reports of the immuno-oncolytic efficiency of TPV in an immuno-competent model. As TPV only replicates in humans and monkeys, this is a significant step forward for the development of other tropism-limited OVs. Poxviruses have been shown to bear oncolytic potential against CRC [21,23-25,38-40]. In fact, several clinical trials have been conducted to evaluate their immuno-oncolytic efficacy against CRC in humans [41-43]. Antiviral immune response [44, 45] and person-to-person transmission are two of the major hurdles in OV therapy. The availability of an entire spectrum of antigenically and serologically distinct oncolytic viruses may enable sidestepping the antiviral response as patients can be treated in series as they develop immunity to each virus utilized. There has been no report of human-to-human transmission of TPV. Hence, oncolytic TPV can be a valuable addition to the OV repertoire.
The safety and ease with which viruses can be produced and maintained at a high titer in the laboratory are desirable features in OVs [46]. Manufacturing constraints may hinder the high titer production of many OVs [47,48]. A high dose of OV is especially required for systemic delivery due to the possibility of a significant portion of the administered OV being neutralized by the pre-existing antibodies, circulating complements, and non-specific uptake of the OVs [44,45]. Besides, virus-mediated toxicities and maximum tolerated dose (MTD) of OVs needs to be determined for a safe and effective OV therapy [49,50]. Hence, the production of high titer OV stock is warranted. Our virus replicates reasonably efficiently in the proper laboratory culture. Notably, OMK cells were found to be highly permissive for high virus titer production. A concentrated TPV/Δ2L/Δ66R/ FliC stock consisting of 1 X 1010 pfu/ml were obtained during this study.
Virus mediated cytolysis can be beneficial for the regression of tumors when implementing oncolytic virotherapy. However, another benefit of OV therapy derives from the potential induction of anti-tumor immunity [51]. The study of OV mechanism of action warrants experimental models that reproduce both the tumor-killing and immune activation effects of these viruses. The most commonly used preclinical models, so far, have been immuno-compromised mice and syngeneic tumor models. Despite differences in the immune system between humans and mice, these models have been useful in elucidating the safety, efficacy, and the interactions of OVs with the host innate immune system [9,52,53]. However, the immuno-deficient models' defective adaptive immune system is unsuitable for understanding OVs' interaction with the entire immune system intact [54]. Therefore, to examine such interactions, immunocompetent models are required. Many OV studies have used syngeneic tumor models, and the results obtained from these studies showed the role of OVs, particularly those armed with immune-stimulatory transgenes, to elicit a host anti-tumor immune response and immunological anti-tumor memory [55- 58]. Our previous study has shown that the recombinant TPV/ Δ2L/Δ66R/FliC resulted in a significant hCRC tumor reduction in nude mice and induced some anti-tumor innate immune activity [9]. In the present study, we used an immuno-competent model to better understand the immune system's participation and interaction, particularly the adaptive system, with TPV/Δ2L/ Δ66R/FliC in the treatment of CRC.
One weakness associated with the preclinical TPV study is that it is not a natural pathogen of rodents and does not replicate in mouse cells. Similar challenges have been shown to be present for other OVs as well [59]. In order to eliminate such an obstacle, we used hCRC xenografts in Balb/C mice. To establish and prevent the immediate rejection of the xenograft, we used immuno-deficient mice. We subsequently reconstituted the immune system in these immuno-deficient Balb/C nude mice by the adoptive transfer of splenocytes from the immunologically matched, normal Balb/C mice which are immuno-competent. However, we first allowed the xenografted tumor volume to surpass 100 mm3 before starting virotherapy and subsequent immune reconstitution of these nude mice. One reason behind this experimental design was that a well-established tumor of such size would be more resilient to immune rejection.
We kept a window of thirteen days between the administration of the virus and the mice's immune reconstitution. The purpose was to allow TPV's initial proliferation in the tumor in a partly compromised immune state. According to Speranza and colleagues, an ideal OV therapeutic approach may warrant an initial immune suppression to facilitate sufficient viral replication to harness the host immune system's power for a durable systemic anti-tumor effect and improved tumor reduction [63].
A single intratumoral injection of TPV/Δ2L/Δ66R/FliC caused a robust reduction of the injected tumors. An almost complete reduction of injected tumors was observed for 80% of the mice in the virus treatment group by the time the experiment was terminated. The ability to produce a curative effect against cancer upon a single dose is a desirable feature of OV therapy and is absent for many OVs that require booster shots to produce sufficient antitumor effect [60]. The TPV/Δ2L/Δ66R/ FliC-mediated reduction of the tumors was found to be more pronounced toward the end of the experiment. When tumor volumes from day 24 to day 38 were compared, a significant reduction of the injected tumor volumes in the virus treatment group was observed at three different time points – on day 32, 36, and 38, compared to the immune-reconstitution control group. During this period, the regression of these virus treated tumors was significant at nearly all time points compared to the vehicle control group. This period of robust tumor regression in the virus treatment group followed the immune reconstitution of the mice and coincided with the time-period required for the development of possible anti-tumor immunity. Thus, these tumor regression time points suggest the involvement of the adaptive immune system against CRC xenografts. This is supported by the robust regression of the tumors in the reconstitution control group. Graft rejection is mediated via T-cell activity, which is absent in athymic nude mice used in this study. Therefore, splenocyte reconstitution from immunologically matched normal mice must have supplied the T cells necessary to mount the rejection response and anti-tumor activity.
Interestingly, a notable anti-tumor effect could also be observed on the non-injected tumors contralateral to the injected tumors. The regression of these tumors was evident and significant compared to the vehicle control group; however, it was not significant compared to the immune-reconstitution control group. The reduction of these contralateral tumors in the TPV/ Δ2L/Δ66R/FliC treated mice may suggest the induction of systemic anti-tumor immunity. Virus infection is known to induce vigorous cytotoxic T cell responses, and the systemic immunity can arise from intratumoral injections of OVs [61]. Previous studies have shown the induction of tumor-specific cytotoxic T cell-mediated immunity following intramural OV inoculation [62].
Conclusion
In conclusion, TPV/Δ2L/Δ66R/FliC caused significant regression of injected tumors in immuno-competent mice. A notable reduction of non-injected tumors contralateral to these injected ones could be observed, suggesting the induction of systemic anti-tumor immunity. Taken together, these results showed that the recombinant TPV/Δ2L/Δ66R/FliC might be a potential candidate for treating CRC in humans and should be explored further for the complete realization of its potential.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J cancer [Internet]. 2015; 136: E359-86.
- Cancer Facts & Figures | American Cancer Society, 2020.
- Board PS and PE. Colorectal Cancer Screening (PDQ®). PDQ Cancer Inf Summ, 2021.
- Atlanta GA. American Cancer Society. Colorectal Cancer Facts & Figures 2022.
- Institute NC. Colorectal Cancer-Cancer Stat Facts, 2022.
- Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Prim, 2015.
- Häfner MF, Debus J. Radiotherapy for Colorectal Cancer: Current Standards and Future Perspectives. Visc Med. 2016; 32: 172-7.
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015; 14: 642– 62.
- Conrad SJ, El-Aswad M, Kurban E, Jeng D, Tripp BC, Nutting C, et al. Oncolytic tanapoxvirus expressing FliC causes regression of human colorectal cancer xenografts in nude mice. J Exp Clin Cancer Res. 2015; 34.
- Aurelian L, Bollino D, Colunga A. The oncolytic virus ΔPK has multimodal anti-tumor activity. Pathog Dis. 2016; 74.
- Wang B, Ogata H, Takishima Y, Miyamoto S, Inoue H, Kuroda M, et al. A novel combination therapy for human oxaliplatin-resistant colorectal cancer using oxaliplatin and coxsackievirus A11. Anticancer Res. 2018; 38: 6121-6.
- Moss B. Poxviridae: the viruses and their replication. In: Fields, B.N., Knipe, D.M., Howley PM, editor. Fields’ Virology. 3rd ed. Philadelphia: Lippincott-Raven. 1996; 2637-71.
- Le Bœuf F, Bell JC. United virus: The oncolytic tag-team against cancer! Cytokine Growth Factor Rev. 2010; 21: 205-11.
- Smith GL, Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983; 25: 21-8.
- Chan WM, McFadden G. Oncolytic Poxviruses. Annu Rev Virol. 2014; 1: 119-41.
- Johnston JB, McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J Virol. 2003; 7: 6093–100.
- de Clercq E. Historical perspectives in the development of antiviral agents against poxviruses. Viruses. 2010; 2: 1322–39.
- Downie AW, Taylor-Robinson CH, Caunt AE, Nelson GS, MansonBahr PE, Matthews TC. Tanapox: a new disease caused by a pox virus. Br Med J. 1971; 1: 363–8.
- Nakano JH. R echerch e Human tanapox in Zaire : clinical and epidemiological observations on cases confirmed by laboratory studies. 1985; 63.
- Nazarian SH, Barrett JW, Stanford MM, Johnston JB, Essani K, McFadden G. Tropism of Tanapox virus infection in primary human cells. Virology. 2007; 368: 32-40.
- O’Leary MP, Warner SG, Kim SI, Chaurasiya S, Lu J, Choi AH, et al. A Novel Oncolytic Chimeric Orthopoxvirus Encoding Luciferase Enables Real-Time View of Colorectal Cancer Cell Infection. Mol Ther oncolytics. 2018; 9: 13-21.
- Francis L, Guo ZS, Liu Z, Ravindranathan R, Urban JA, Sathaiah M, et al. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget. 2016; 7: 22174-85.
- Yoo SY, Bang SY, Jeong SN, Kang DH, Heo J. A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget. 2016 ; 7: 16479-89.
- Haddad D, Chen N, Zhang Q, Chen CH, Yu YA, Gonzalez L, et al. A novel genetically modified oncolytic vaccinia virus in experimental models is effective against a wide range of human cancers. Ann Surg Oncol. 2012; 19.
- Sathaiah M, Thirunavukkarasu P, O’Malley ME, Kavanagh MA, Ravindranathan R, Austin F, et al. Oncolytic poxvirus armed with Fas ligand leads to induction of cellular Fas receptor and selective viral replication in FasR-negative cancer. Cancer Gene Ther 2012 193. 2011; 19: 192-201.
- Liu Z, Yang Y, Zhang X, Wang H, Xu W, Wang H, et al. An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation. Hum Gene Ther. 2017; 28: 667–80.
- Grossardt C, Engeland CE, Bossow S, Halama N, Zaoui K, Leber MF, et al. Granulocyte-macrophage colony-stimulating factorarmed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum Gene Ther. 2013; 24: 644-54.
- Yin L, Zhao C, Han J, Li Z, Zhen Y, Xiao R, et al. Antitumor effects of oncolytic herpes simplex virus type 2 against colorectal cancer in vitro and in vivo. Ther Clin Risk Manag. 2017; 13: 117-30.
- Stephenson KB, Barra NG, Davies E, Ashkar AA, Lichty BD. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012; 19: 238-46.
- Li J, O’Malley M, Sampath P, Kalinski P, Bartlett DL, Thorne SH. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia. 2012; 14: 1115-21.
- McQuiston JR, Parrenas R, Ortiz-Rivera M, Gheesling L, Brenner F, Fields PI. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flpA from Salmonella. J Clin Microbiol. 2004; 42: 1923-32.
- Andersen-Nissen E, Smith KD, Bonneau R, Strong RK, Aderem A. A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J Exp Med. 2007; 204: 393-403.
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galán JE, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006; 103: 12487- 92.
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001; 167: 1882-5.
- Eaves-Pyles T, Murthy K, Liaudet L, Virág L, Ross G, Soriano FG, et al. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 2001; 166: 1248-60.
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Tolllike receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003; 170: 5165-75.
- Mediratta S, Essani K. The replication cycle of tanapox virus in owl monkey kidney cells. Can J Microbiol [Internet]. 1999; 45:92-6.
- Francis L, Guo ZS, Liu Z, Ravindranathan R, Urban JA, Sathaiah M, et al. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget. 2016; 7: 22174-85.
- Badrinath N, Jeong Y Il, Woo HY, Bang SY, Kim C, Heo J, et al. Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes. Int J Pharm. 2018; 552: 437-42.
- Ferguson MS, Chard Dunmall LS, Gangeswaran R, Marelli G, Tysome JR, Burns E, et al. Transient Inhibition of PI3Kδ Enhances the Therapeutic Effect of Intravenous Delivery of Oncolytic Vaccinia Virus. Mol Ther. 2020; 28: 1263-75.
- Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UNM, Ramalingam L, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015; 23: 202-14.
- Downs-Canner S, Guo ZS, Ravindranathan R, Breitbach CJ, O’Malley ME, Jones HL, et al. Phase 1 Study of Intravenous Oncolytic Poxvirus (vvDD) in Patients With Advanced Solid Cancers. Mol Ther. 2016; 24: 1492.
- Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol Ther. 2015; 23: 1532.
- Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007; 15: 660–5.
- Jennings VA, Ilett EJ, Scott KJ, West EJ, Vile R, Pandha H, et al. Lymphokine-activated killer and dendritic cell carriage enhances oncolytic reovirus therapy for ovarian cancer by overcoming antibody neutralization in ascites. Int J cancer. 2014; 134: 1091- 101.
- Ungerechts G, Bossow S, Leuchs B, Holm PS, Rommelaere J, Coffey M, et al. Moving oncolytic viruses into the clinic: clinicalgrade production, purification, and characterization of diverse oncolytic viruses. Mol Ther Methods Clin Dev. 2016; 3: 16018.
- Shekarian T, Sivado E, Jallas AC, Depil S, Kielbassa J, JanoueixLerosey I, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med. 2019; 11.
- Kim KH, Dmitriev IP, Saddekni S, Kashentseva EA, Harris RD, Aurigemma R, et al. A Phase I Clinical Trial of Ad5/3-Δ24, a Novel Serotype-Chimeric, Infectivity-Enhanced, Conditionally-Replicative Adenovirus (CRAd), in Patients with Recurrent Ovarian Cancer. Gynecol Oncol. 2013; 130: 518.
- Machiels JP, Salazar R, Rottey S, Duran I, Dirix L, Geboes K, et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J Immunother cancer. 2019; 7.
- Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol Ther. 2017; 25:2620-34.
- Marelli G, Howells A, Lemoine NR, Wang Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front Immunol. 2018; 9:1-8.
- Alvarez-Breckenridge CA, Yu J, Price R, Wojton J, Pradarelli J, Mao H, et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat Med. 2012; 18:1827-34.
- Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007; 67: 9398-406.
- Belizário JE. Immunodeficient Mouse Models: An Overview. Open Immunol J. 2009; 2: 79-85.
- Benencia F, Courrèges MC, Conejo-García JR, Mohammed-Hadley A, Coukos G. Direct vaccination with tumor cells killed with ICP4-deficient HSVd120 elicits effective antitumor immunity. Cancer Biol Ther. 2006; 5: 867-74.
- Benencia F, Courrèges MC, Conejo-García JR, Mohamed-Hadley A, Zhang L, Buckanovich RJ, et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther. 2005; 12: 789-802.
- Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific antitumor immunity. Hum Gene Ther. 1999; 10: 385-93.
- Todo T, Martuza RL, Dallman MJ, Rabkin SD. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 2001; 61: 153- 61.
- Corey L, Spear PG. Infections with herpes simplex viruses (2). N Engl J Med. 1986; 314: 74957.
- Yang G, Meng X, Sun L, Hu N, Jiang S, Sheng Y, et al. Antitumor effects of a dual cancer-specific oncolytic adenovirus on colorectal cancer in vitro and in vivo. Exp Ther Med. 2015; 9: 327-34.
- Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y, Nishiyama Y. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008; 50: 185-96.
- Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001; 98: 6396- 401.