Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 2
The first of laparoscopic gastrectomy with D2 lymph dissection in our experience
Tillyashaykhov Mirzagaleb N; Adilkhodjaev Askar A; Khudoyarov Sanjar S; Rakhimov Okiljon A; Khusnuddinov Nizomuddin Z; Yunusov Seydamet Sh*
Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Tashkent, Uzbekistan.
*Corresponding Author : Yunusov Seydamet Sh
Republican Specialized Scientific and Practical
Medical Center of Oncology and Radiology, Tashkent, Uzbekistan.
Email: dr.syu1990@gmail.com
Received : Jan 25, 2022
Accepted : Mar 04, 2022
Published : Mar 09, 2022
Archived : www.jjgastro.com
Copyright : © Seydamet Sh Y (2022).
Abstract
The article presents the first laparoscopic gastrectomy performed at the Republican specialized Scientific and practical Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan of a 56-year-old patient with a diagnosis of Adenocarcinoma G2 of the body of stomach. The operation was performed laparoscopically. Advantages of this operation are minimal trauma, radicalism, good ergonomics, minimal blood loss, a smooth postoperative period and a rehabilitation period.
Keywords: gastric adenocarcinoma; laparoscopic gastrectomy; esophageal entero anastomosis.
Citation: Mirzagaleb NT, Askar AA, Sanjar SK, Okiljon AR, Seydamet Sh Y, et al. The first of laparoscopic gastrectomy with D2 lymph dissection in our experience. Japanese J Gastroenterol Res. 2022; 2(4): 1066.
Introduction
Mortality from oncological diseases ranks second, after diseases of the cardiovascular system (63.8%) and it is 9.6%, in accordance with world statistics [3]. In the Republic of Uzbekistan, at the end of 2019, 103,063 cancer patients were registered with the dispensary [1]. The first place is occupied by breast cancer, which accounted for 19.4%, and gastric cancer accounted for 3.8% of all malignant neoplasms [1,3]. The first successful resection for gastric cancer was performed by Theodor Billroth in 1881. This method is considered the only radical method for the treatment of gastric cancer. The treatment of gastric cancer has been and remains surgical, including complete removal of the tumor and areas of its possible regional metastasis [2]. A real revolution in endoscopic technology is associated with the development and implementation of fiber-optic technologies at the turn of the 1970s and 1980s and the creation of a matrix capable of transforming a video signal to transmit it to monitor. To date, the general advantages of laparoscopic access are obvious and do not require proof [5]. Back in 1990, a group of Japanese scientists led by T. Okusa showed that the number and proportion of metastatic lymph nodes (LN) are directly related to 5-year survival rates, which underlie the classification of the International Anti-Cancer Union UICC/TNM (Union International Against Cancer), the seventh edition of which has been in force since 2009 [4].
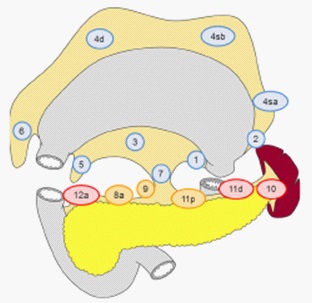
Traditionally, radical surgery for cancer of various localizations includes the obligatory monoblock sheath-fascial removal of regional fat along with lymphatic vessels and nodes. K.P. Sapozhkov in 1945 put forward the idea of a principled gastrectomy with extended lymphadenectomy in gastric cancer, calling it “the ultimate radical operation” [2]. The works of J. Soga (1979) and Y. Kodama (1981) showed a significant improvement in survival results with an increase in the volume of lymph dissection (LD). Total resection of the stomach is a resection of the stomach, including the cardiac and pyloric parts of the stomach [6]. LD with D1 includes removal of perigastric LNs (groups 1–6), LD with D2 removal of groups 7–11 is added to removal of 1–6, LD with D3 additional excision of LNs of groups 12–16 is performed (Figure 1).
The first laparoscopic subtotal resection of the stomach for gastric cancer was performed by Seigo Kitano in 1991; since then, minimally invasive interventions for gastric cancer have become widespread throughout the world [6]. A study in the National Clinical Database (NCD) comparing gastric outcomes with laparoscopic distal gastrectomy and open distal gastrectomy concluded that neither postoperative mortality nor deaths with gastric cancer were significantly higher for laparoscopic surgery than for the conventional open approach [5]. Also in a study comparing Laparoscopic-assisted total gastrectomy and open total gastrectomy, esophagogastric anastomosis failure occurred in 0-4% and 2-3%, respectively [6]. In connection with the foregoing, the use of videolaparoscopic interventions for gastric cancer is promising, which is by far the safest and meets all the requirements used in oncology. Below is our first experience with laparoscopic gastrectomy with D2 LD.
Patient B, 58 years old, was admitted to the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology on October 26, 2019 with a diagnosis: Cancer of the stomach: ulcerative infiltrative form with localization in the antrum and body of the stomach (the result of a histological study: adenocarcinoma G2). At the time of admission, the patient considered herself for 3 months, when she first noted pain in the epigastric region, weight loss, and general weakness. Ulcer history at the time of hospitalization was denied. In the dynamics noted the progression of symptoms. In this connection, she turned to the RSNPMCOR, where a gastroscopic examination in the antrum and body of the stomach revealed a formation measuring 4 X 3 cm, with moderate infiltration around, a biopsy was taken. Histology results №3153: G2 adenocarcinoma. There are no data for distant metastases in 3-phase CT examination. Upon admission, the general condition of moderate severity, skin and visible mucous membranes of normal color, body temperature 36.5°C. There are no rashes on the skin and visible mucous membranes. Peripheral lymph nodes are not enlarged.
Vesicular breathing is heard in the lungs on both sides, there are no wheezing. Respiration rate 18 per minute. During auscultation of the heart, the tones are muffled, rhythmic, the heart rate is 86 per minute. Blood pressure 120/80 mmHg. Tongue moist, covered with white coating. The abdomen is of normal shape, symmetrical, participates in the act of breathing, soft, moderately painful in the epigastric region. There are no peritoneal symptoms and muscle tension of the anterior abdominal wall. The liver and spleen are not enlarged. There is no peripheral edema. Diuresis is free, regular. Stool regular, normal color.
Blood group and Rh factor: B (III) Rh + (positive).
Common blood test from 10/27/2019: hemoglobin 114 g/l, erythrocytes 3.5 X 1012/l, leukocytes 7.0 X 109 /l.
Common urin test from 10/27/2019: acid reaction, protein and glucose are negative, epithelium are 0-1-2-3/1, leukocytes are 1-2-3/1, salts are +. Biochemical blood test from 10/27/2019: alanine aminotransferase 30.0 U/L, aspartate aminotransferase 26.0U/L, bilirubin 8.3 mmol/l, urea 6.03 mmol/l, creatinine 74.0 mmol/l, total protein 71.0 g/l. Coagulogram from 10/27/2019: fibrinogen 2.60 g/l, prothrombin index 94%, international normalized ratio 1.17, hematocrit 36%, thrombotest V, blood coagulation time 3’10”-4’00”. Ultrasound investigation from 10/27/2019: There is no free fluid in the abdominal cavity, liver, pancreas, spleen has no visible pathology, lymph nodes of the liver, paraortal, bifurcation are not visualized.
MRI of the abdominal organs with 3-phase contrast: In the antrum of the stomach, closer to the body, there is a volumetric formation, 4.3-3.2 cm in size, with moderate stenosis of the gastric outlet of paragastric lymph nodes of the gate of the liver with a maximum diameter of 5 mm. The patient was consulted by a cardiologist, a chemotherapist, a radiologist, given the lack of data for a locally advanced of process, it is more expedient to start treatment with a surgical. On 11/1/2019, to patient underwent the operation “Laparoscopic gastrectomy with D2 lymph node dissection” for the first time in our clinic. Postoperative diagnosis: "Cancer of the body of the stomach (adenocarcinoma G2) T2N0M0".The operation was performed by one surgical team, consisting of an operator, first and second assistants (Figure 2,3).
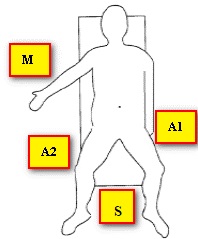
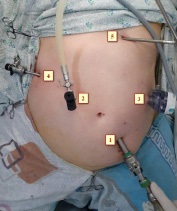
The patient was in the Trendelenburg position, the operating surgeon was located between the patient's legs, the first assistant on the left, the second assistant on the right of the patient. The monitor is located at the head of the patient. Such an arrangement of the operating team allowed free visualization and manipulation in all parts of the abdominal cavity. To perform surgical approaches, we used: one 10 mm, one 12 mm, and three 5 mm trocars, the location of the trocars is shown in (Figure 4).
The operation was performed under general anesthesia, standard 5-10 mm in diameter and 360 mm long, endoscopic instruments from Carl Storz (Germany) were used as surgical instruments using the endovideo stand of the same name. In our observation, were used 300 10 mm optics. For tissue dissection and coagulation, a Harmonic ultrasonic dissector from Johnson & Johnson was used. For mechanical connection of organs and tissues, a linear stapling device EndoGia 60 mm Tri Stapler technology manufactured by Сovidien (France) and a cassette depth of 4.8 mm was used. For traction of the left lobe of the liver, in order to better visualize the cardioesophageal zone, we used the Nadhanson ecorter. Intra-abdominal pressure was maintained at the level of 14-15 mmHg.
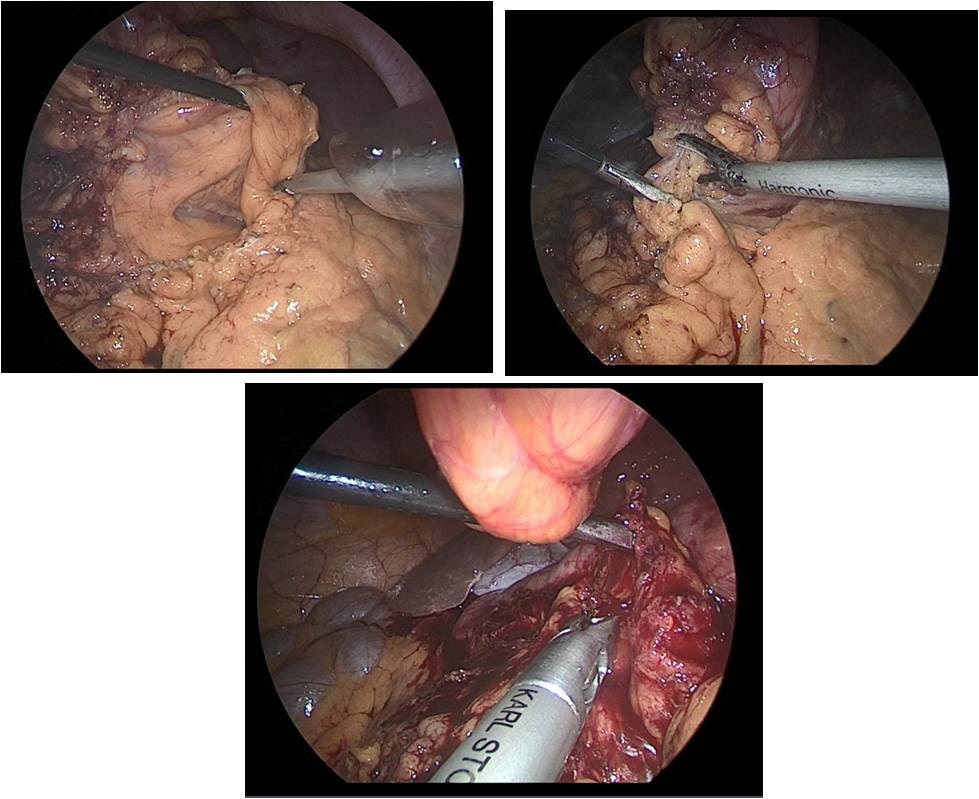
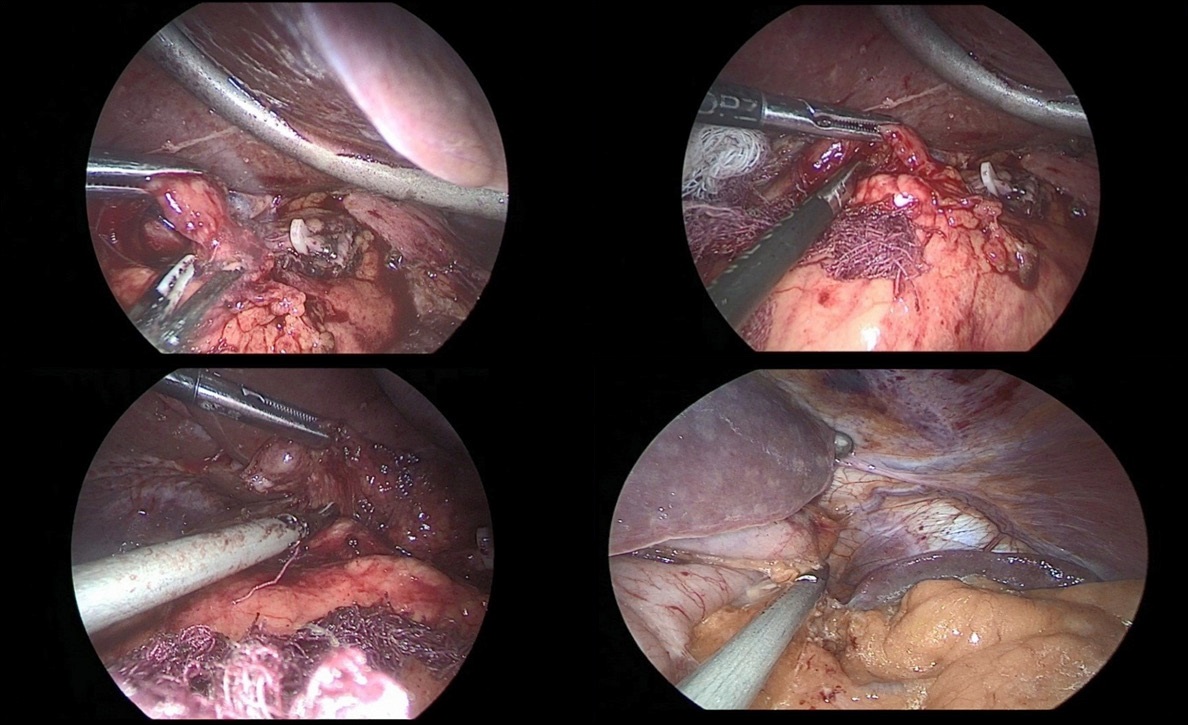
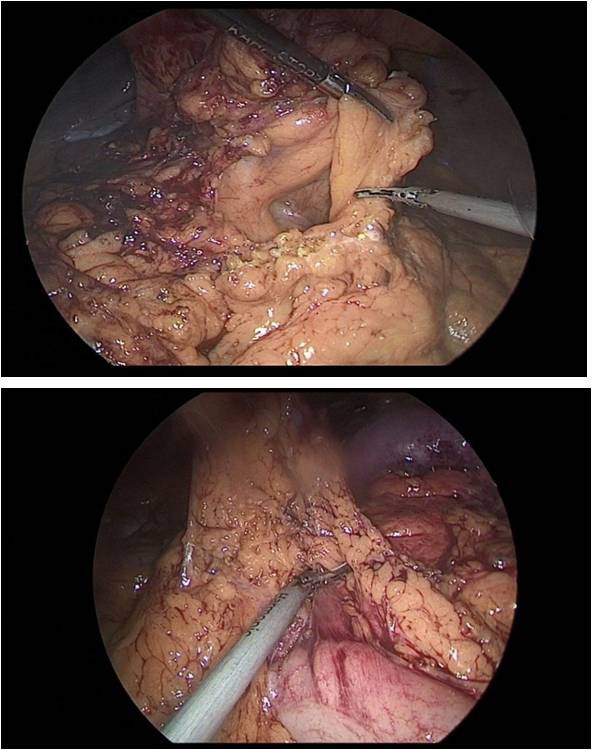
So, after the installation of the optical trocar, and the injection of carbondioxyde to peritoneum, working trocars were introduced and a laparoscopic revision of the abdominal cavity was carried out for the presence of micrometasations of the parietal peritoneum, liver and mesentery of the small and large intestines. After that, was performed the mobilization of the greater curvature of the stomach (Figure 5a,b,c).
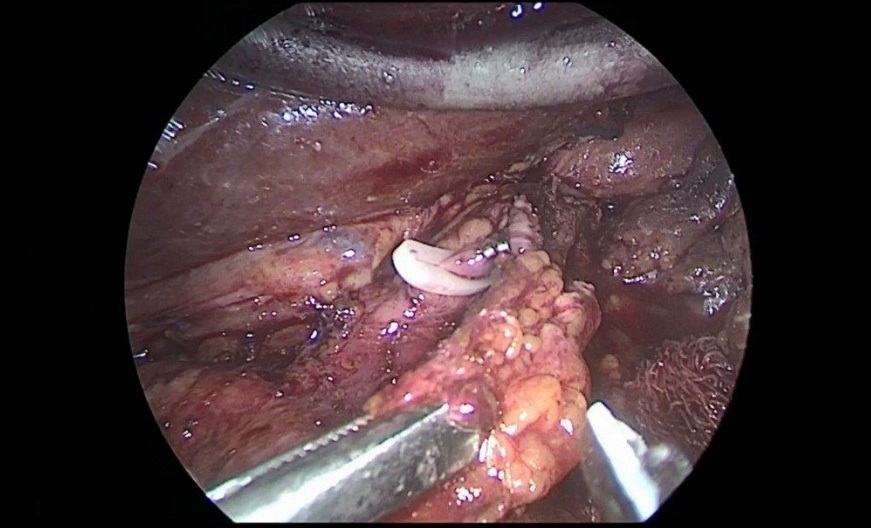
The stomach is mobilized to pyloric sphincter. Next, was performed mobilization along the lesser curvature of the stomach (Figure 8). For this, the lesser omentum was resected, lymph node dissection of I, III, V, VII groups of lymph nodes. The left gastric artery was clipped (Figure 6a,b) and transected (Figure 7).
After mobilization of the stomach along the greater and lesser curvature of the stomach, the distal part of the stomach was transected at the level of the pyloric sphincter with the EndoGia 60 mm apparatus (Figure 8).
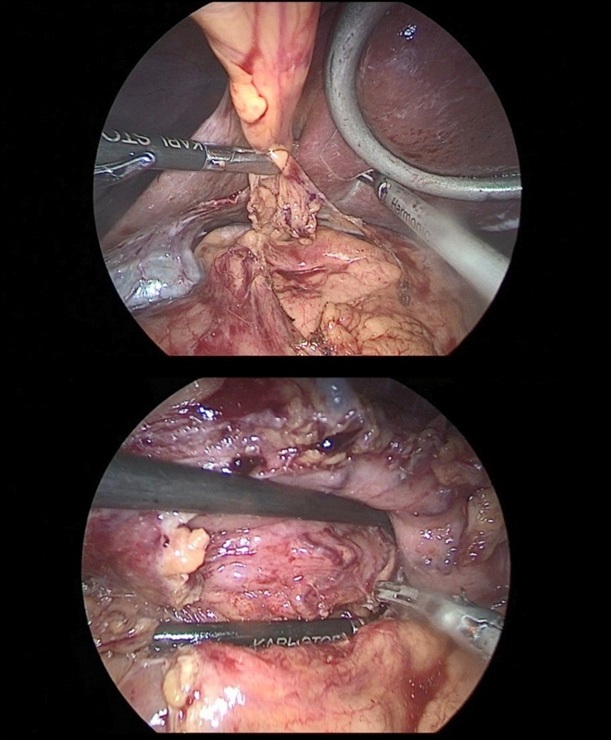
After crossing the pyloric sphincter, laparoscopic lymph node dissection of the VII, VIIIa, IX, XI, XIIa groups of lymph nodes was performed (Figure 9 a, b, c, d).
Then performed resection of the greater omentum with lymph dissection II, IV, VI groups of lymph nodes (Figure 10a,b).
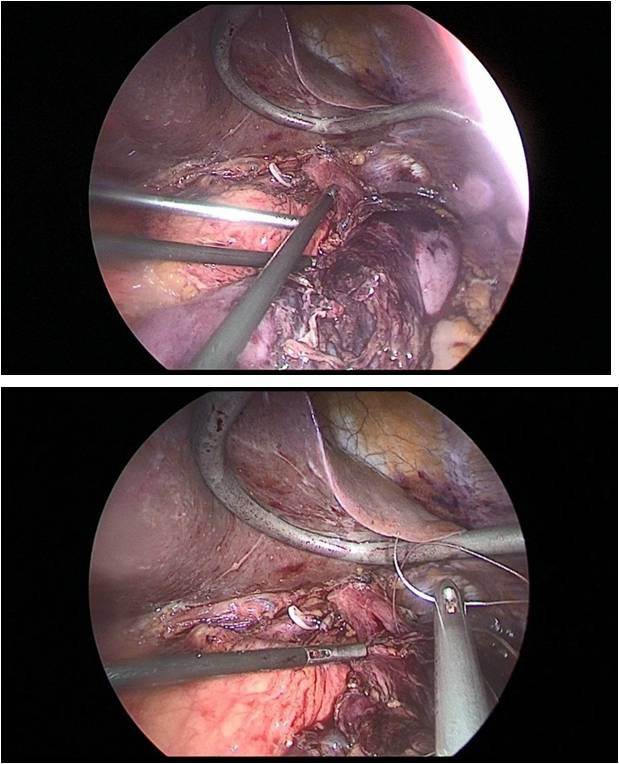
Further, 30 cm away from the ligament of Treitz, the jejunum was transected to apply esophagojejunal anastomosis, then the abdominal part of the esophagus was mobilized, with a pursestring suture with a Vicryl 2.0 thread on the adventitia of the esophagus (Figure 11a,b).
This completed the laparoscopic stage of the operation. Next, a minilaparotomy up to 6 cm long was performed along the white line of the abdomen, retreating 5 cm below the xiphoid process. The stomach was transected at the level of the cardioesophageal fold, after which the jejunum was brought to the abdominal esophagus through the mesocolon window. Then, a circular stapler was inserted through the free edge of the transected jejunum, and a hole was made at a distance of 10 cm from the resected edge along the antimesenteric, into which a circular stapler was inserted and end-to-side esophagojejunostomy was applied (Figure 12).
A linear stapler is applied to the free edge of the jejunum. The next step was to form an intra intestinal Y-shaped anastomosis at a distance of 40 cm from the esophagus-entero anastomosis. The last step, in order to prevent the formation of internal hernias, was sutured defects in the mesentery of the small intestine. And for the purpose of decompression of the intestine and early enteral nutrition, a gastric tube with a diameter of 18 F was inserted behind the entero-enteroanastomosis. The macropreparation was removed through minilaparotomy access. The abdominal cavity is sutured with interrupted sutures. Intraoperative blood loss was 90-100 ml, the duration of the operation was 540 minutes.
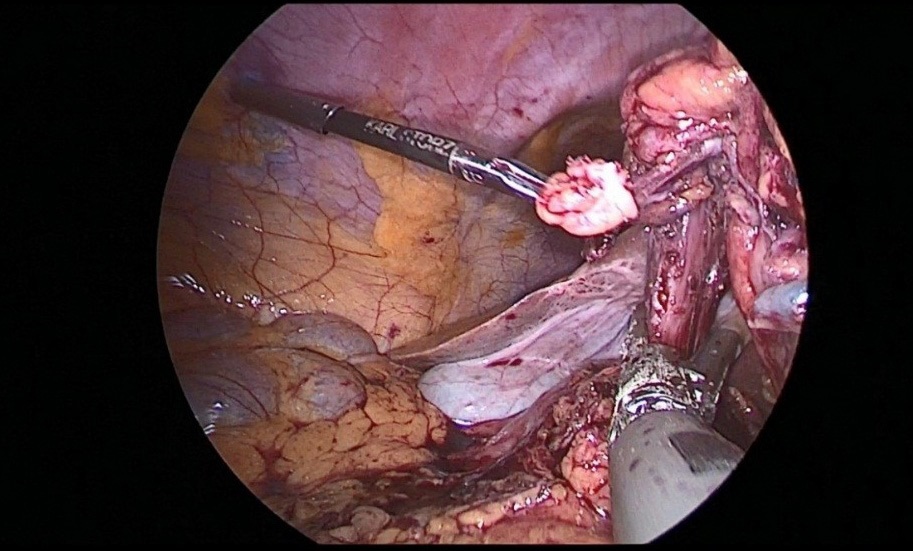
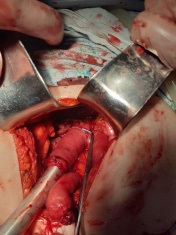
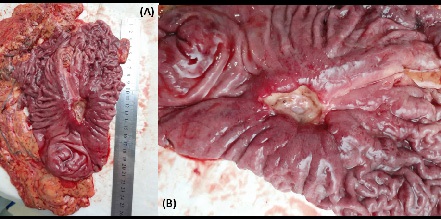
Removed macropreparation: stomach, in the body of the stomach, a tumor with decay elements, 4 X 4 cm in size, with undermined and infiltrated edges (Figure 13a,b). Histological conclusion: Adenocarcinoma G-2 (Figure 14).
The postoperative period was uneventfully, a course of antibacterial, infusion, antiulcer therapy was carried out. The patient was activated on the 2nd day after the operation. On the 2nd day, intestinal peristalsis was auscultated, feeding through a tube was started. On the 5th day after the control X-ray examination, the gastric tube was removed. On the 4th day there was an independent stool, the drainage of the abdominal cavity was removed on the 6th day after the operation. On the 9th day the patient was discharged from the hospital in a satisfactory condition. The patient underwent adjuvant chemotherapy in the postoperative period, the observation continues, there are no data for a relapse of the disease. The demonstrated clinical example showed that minimally invasive surgery for gastric cancer is one of the complex and multicomponent sections of surgery. Performing laparoscopic gastrectomy requires both specific endoscopic skills in manipulation and the use of endosurgical instruments, as well as adherence to oncological standards of lymph node dissection, and the use of intracorporeal anastomosis in gastric cancer surgery, makes it possible to perform the operation with minimal trauma, thereby reducing the invasiveness of surgical aggression.
References
- Tillyashaykhov MN. Sostoyaniye onkologicheskoy sluzhby v Respublike Uzbekistan. Materialy X s’yezda onkologov i radiologov stran SNG i Yevrazii. Sochi, Rossiya. 23-25 aprel’ 2018.
- AM Karachun, VA Kashchenko, YuV Pelipas. Technical aspects of laparoscopic gastrectomy for gastric cancer. Nauchno-prakticheskiy zhurnal federal’nogo mediko-biologicheskogo agentstva. Klinicheskaya bol’nitsa. №2 (16) 2016; 6-20.
- Gastric cancer: ESMO clinical practice Guidelines. Ann Oncol. 2016; 27: 38-49.
- Japanese gastric cancer treatment guidelines. 2014; 4: 226.
- K Yoshida, M Honda, H Kumamaru, Y Kodera, Y Kakeji, N Hiki, et al. Surgical outcomes of laparoscopic distal gastrectomy compared to open distal gastrectomy: A retrospective cohort study base donanation wide registry database in Japan. 2018; 690- 696.
- K Kataoka, H Katai, J Mizusawa, H Katayama, K Nakamura, S Morita, et al. Non-Randomized Confirmatory Trial of Laparoscopy-Assisted Total Gastrectomy and Proximal Gastrectomy with Nodal Dissection for Clinical Stage I Gastric Cancer. 2019; 711- 780.