Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
miRNA-155-5p and miRNA-let-7a-5p expression controlled by Helicobacter pylori
Santos Mônica P1; Pereira Jéssica N1; Almeida Laine A1; Labio Roger W1; Smith Marilia AC2; Barbosa Mônica S3; Payão Spencer LM1; Rasmussen Lucas T1*
1 Faculdade de Medicina de Marília (FAMEMA), Marília, São Paulo, Brazil.
2 Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil.
3 Universidade Federal de Goiás (UFG), Goiânia, Goiás, Brazil.
*Corresponding Author: Lucas Trevizani Rasmusse
Genetics laboratory, Lourival Freire, 240, Bairro Fragata, CEP 17519-050, Marília, São Paulo, Brazil.
Email: lucasrasmussen@gmail.com
Received: Dec 30, 2021
Accepted : Feb 27, 2022
Published: Mar 04, 2022
Archived: www.jjgastro.com
Copyright: © Rasmussen LT (2022).
Abstract
Introduction: Gastric diseases, including Gastric Cancer (GC), are frequently caused by Helicobacter pylori infection and its virulence factors, such as the cagA and cagT genes. Along with H. pylori infection, dysregulation of specific microRNAs (miRNAs) also appears to contribute to the development of this diseases. Therefore, this study investigated miRNA-155-5p and miRNA-let-7a-5p expression, taking into consideration virulence factors of H. pylori to understand their relationship with GC development.
Methods: Polymerase Chain Reaction (PCR) was employed to detect H. pylori and the virulence factors. Real-Time-qPCR was used to evaluate miRNA expression. A total of 208 gastric biopsy samples were obtained from patients with gastric symptoms.
Discussion: According to the histological analysis, they were divided into groups: Control, Gastritis, and Gastric Cancer. H. pylori was detected in 43.7%, and cagA/cagT genes in 30.7% and 38.4%, respectively. Further, H. pylori seems to influence the miRNA-155-5p expression, which showed an increased expression in the Gastritis (p=0.0015*) and GC (p=0.0001*) groups positive for H. pylori. The virulence factor cagT gene also promoted an increase expression of this miRNA in patients with GC (p=0.0222*). On the other hand, the GC group had a low expression of miRNA-let-7a-5p (p<0.0001*), considering or not H. pylori infection.
Conclusion: Our results indicated that H. pylori and virulence factor, specifically the cagT gene, influence the expression of miRNA155-5p and miRNA-let-7a-5p, as an important mechanism involved in the inflammatory process, contributing to the development of Gastritis and GC. Furthermore, H. pylori infection increases the risk for the development of gastric diseases.
Keywords: gene expression; stomach neoplasms; miRNA-155a-5p; miRNA-let-7a-5p; Helicobacter pylori.
Citation: Mônica PS, Jéssica NP, Laine AA, Roger WL, Lucas TR, et al. miRNA-155-5p and miRNA-let-7a-5p expression controlled by Helicobacter pylori. Japanese J Gastroenterol Res. 2022; 2(4): 1064.
Introduction
Helicobacter pylori infection in the gastric epithelium is one of the most prevalent infections in the world. Generally, the infection is acquired in early childhood and prevails in approximately half of the world population, which may or may not have symptoms [1,2]. H. pylori is characterized as a gram-negative and microaerophilic bacterium, which colonizes the gastric epithelium and activates the host’s immune system, leading to the expression of proinflammatory cytokines. The recruitment of immune cells and the high expression of these cytokines lead to an intense inflammatory process, favoring a possible carcinogenic process [3,4]. Moreover, H. pylori has specific characteristics, such as virulence factors, like cagA and cagT, which seem to further contribute to the development of pathologies associated with its colonization. The cagA gene is located in a segment of DNA, cag pathogenicity island (PAI) in region 5’ (cagI) and was the first specific gene identified in the bacterium and encodes the cagA protein, frequently studied for its pathogenic effects. The cagT gene is located in the cagII region, on cag pathogenicity island, and its protein acts mainly in inflammatory processes. Thus, cagT has been associated with poor prognosis. Taking these factors into account, H. pylori has been associated with the development of several gastric diseases, including GC [5,6].
GC is a multifactorial and complex disease. Although its exact etiology has not been clarified yet, colonization of the gastric epithelium by H. pylori, as well as environmental factors such as diet and smoking along with genetic factors seem to contribute to its development. Among genetic factors, dysregulation of specific microRNAs (miRNAs) may be associated with GC development [7-9]. miRNAs are classified as oncogenic or tumor suppressor. These small non-coding RNAs regulate gene expression and participate in important processes in tumor development, including proliferation, invasion, and angiogenesis, besides acting in the cell growth process. All of these factors indicate their importance in cancer development and progression [7,10]. Furthermore, studies have shown that dysregulation of specific miRNAs could be further influenced by colonization of H. pylori in the gastric mucosa [11,12].
miRNA-155-5p is one of the miRNAs that play important roles in different cardiovascular and inflammatory diseases as well as cancer [13,14]. Nevertheless, it has considerable actions on the immune system, being able to control the intensity of the inflammatory response, and is important in vital biological processes. In the gastrointestinal tract, abnormal levels of this miRNA expression have been demonstrated, especially during H. pylori infection, and are still considered an important factor in the regulation of T cells, as an attempt to eradicate the bacterium. According to Wan et al. (2016) [15], miRNA-155-5p is highly expressed in T cells, macrophages, and gastric epithelial cells during bacterium colonization. On the other hand, miRNAlet-7a-5p has been identified as a tumor suppressor. Studies have shown that high miRNA-let-7a expression can inhibit tumor cell invasion, proliferation, and migration in the GC [16,17]. At high levels of expression, it is capable of inhibiting the tumor cell cycle, although generally its expression is low in GC. In addition, Matsushima et al. (2011) [18] reported a decrease in miRNA-let-7a expression in H. pylori-induced inflammatory process.
As previously mentioned, H. pylori and miRNAs have been widely studied for their participation in the development of different types of cancer. However, few studies in the literature have analyzed the association between these two miRNAs and the virulence factors, especially cagA and cagT of H. pylori, in the development of CG. Therefore, in this study, we evaluated miRNA-155-5p and miRNA-let-7a-5p expression, which are considered important in inflammation and the carcinogenic process, considering or not the presence of H. pylori and its virulence factors, cagA and cagT, to understand their importance in the development of GC.
Materials and methods
Gastric biopsy samples and patients
Two hundred and eight samples of gastric biopsies were obtained from the region of the antrum of the stomach from patients with peptic diseases who had undergone an endoscopic investigation at two hospitals located in the interior of São Paulo (Brazil) and at the Federal University of Goiás and Federal University of São Paulo. All samples were submitted to histopathological analysis based on the Sydney and Lauren’s classification System [19], for division into groups: Control, Gastritis, and GC. We analyzed 60 patients (12♂/38♀, mean age 56 ± 15 years) with healthy gastric mucosa (Control group); 101 (46♂/55♀, mean age 54 ± 17 years) with Chronic Gastritis (Gastritis group), and 47 (16♂/31♀, mean age 56 ± 9 years) with GC. However, for the analysis of the expression of miRNA-155-5p, only one hundred and ninety seven samples were analyzed, following the same standard established for miRNA-let-7a-5p. Patients who received antimicrobial or anti-inflammatory treatment for at least 30 days prior to the examination were excluded from the study. All the patients who participated received and signed a consent form to participate and the study was approved by the Ethics Committee (Case Number 1.119.830) of the Universidade do Sagrado (USC), Bauru, SP, Brazil.
DNA extraction and H. pylori detection
DNA extraction was performed using the Qiagen QiaAmp Kit (Cat. No. 51304; Qiagen, Germany) following the manufacturer’s instructions. PCR was employed to detect H. pylori and virulence factors from the cagA and cagT genes. Details about the oligonucleotides and specific conditions are described in (Table 1). The diagnosis of H. pylori and its virulence factors was obtained by electrophoresis. All fragments were viewed on 2.5% agarose gel stained with ethidium bromide and photographed in a transilluminator on the α Imager 2200 image capture system [2].
Table 1: Information about H. pylori Detection and cagA and cagT genes.
Primers |
Genes |
Primer sequence (5’ - 3’) |
Amplicons |
PCR Conditions |
Reference |
Hpx1 |
16SrRNA |
CTGGAGARACTAAGYCCTCC |
150pb |
40 cycles: |
[38] |
Hpx2 |
GAGGAATACTCATTGCGAAGGCGA |
|
|
||
Cag1 |
cagA |
ATGACTAACGAAACTATTGATC |
232pb |
40 cycles: |
[2] |
Cag2 |
CAGGATTTTTGATCGCTTTATT |
|
|
||
cagT - F |
cagT |
CCATGTTTATACGCCTGTGT |
301pb |
35 cycles: |
[39] |
cagT - R |
CATCACCACACCCTTTTGAT |
|
|
R = A or G and Y = C or T.
RNA extraction, cDNA synthesis, and real-time-qPCR
The biopsy fragments collected for RNA extraction were stored in RNAlater® Tissue Collection (Ambion, Woodlands, TX, USA) and kept at -20°C. The miRNeasy® Mini Kit 50 (Qiagen, cat. No 217004) was used for extraction. The quantification of the extracted RNA was performed using Nanodrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA). The Complementary DNA (cDNA) synthesis for miRNA analysis was performed using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems™, USA). Both techniques were performed following the manufacturer’s instructions. Expression levels was determined by the qPCR technique in the ABI Prism 7500 Fast Sequence Detection System using the assays hsa-miR-155-5p (002623) and hsa-let-7a-5p (000377). The RNU6B (Hs001093) and RNU48 (Hs001006) were employed for normalization, and all measurements were made in duplicate. Relative quantification was calculated using the 2-ΔΔCt method [20].
Statistical analysis
Results with p<0.05 were considered significant. The GraphPad Prism 5 program was used for data analysis. ANOVA, TwoTailed Student’s t-test, and Fisher’s Exact Test were employed.
Results
The first analysis performed in this work was to detect H. pylori, which was found in 91 samples (43.7%). Among the positive samples, the cagA gene was detected in 28 (30.7%) and the cagT gene in 35 (38.4%). The frequency of detection of the H. pylori, ORs, the 95% CI, and the p value as well as the frequency of the virulence factors in Control, Gastritis, and GC groups are described in detail in (Tables 2 and 3), respectively. In agreement with previous studies, the bacterium is found most prevalently in patients with chronic gastritis and GC. The results seems to confirm the influence of H. pylori presence and the development of gastric diseases [21,22].
Table 2: H. pylori detection frequency in the Control, Gastritis, and Gastric Cancer groups. Hp: Helicobacter pylori; GC: Gastric Cancer; n: number of samples; * statistically significant; * 1: Control vs Gastritis; * 2: Control vs GC.
|
Control (n = 60) |
Gastritis (n = 101) |
GC (n = 47) |
Hp - |
49 (81.9%) |
52 (51.5%) |
16 (34.1%) |
Hp + |
11 (18.1%) |
49 (48.5%) |
31 (65.9%) |
OR (95% CI), p |
|
23.8 (0.11-0.51), p=0.0002 * 1 |
0.11 (0.04-0.28), p<0.0001 * 2 |
Table 3: Frequency of cagA and cagT Virulence Factors in patients infected by H. pylori. Hp: Helicobacter pylori; GC: Gastric Cancer; n: number of samples.
Virulence Factor |
Groups |
||||
|
Control (n = 11) |
Gastritis (n = 49) |
GC (n = 31) |
||
|
cagA |
pos. |
3 (27.3%) |
17 (34.7%) |
8 (25.8%) |
|
neg. |
8 (72.7%) |
29 (65.3%) |
23 (74.2%) |
|
Hp + |
|||||
|
cagT |
pos. |
5 (45.4%) |
23 (47%) |
7 (22.6%) |
|
neg. |
6 (54.6%) |
19 (53%) |
23 (77.4%) |
|
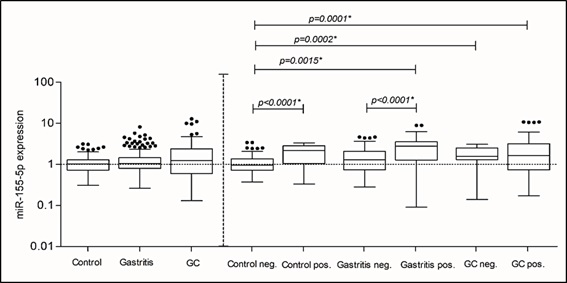
For both miRNAs, the analyses were performed in two parts: in the first, the groups were analyzed without the presence of H. pylori being taken into account. Then, we performed a more refined analysis, in which the three groups were subdivided for the presence or absence of H. pylori (Positive and Negative groups). The Control negative group consisted only of patients with healthy gastric mucosa and no colonization by the bacterium. For miRNA-155-5p, as described above, in the first analysis (Control vs. Gastritis vs. GC), in which the presence of H. pylori was disregarded, no statistically significant difference was found (Figure 1). However, when considering the presence of H. pylori, significant differences were found comparing the Control negative group with: Control, Gastritis, and GC positives for H. pylori groups, and compared to the Cancer negative group. We also found statistically significant differences between the Gastritis negative and positive groups (Figure 1).
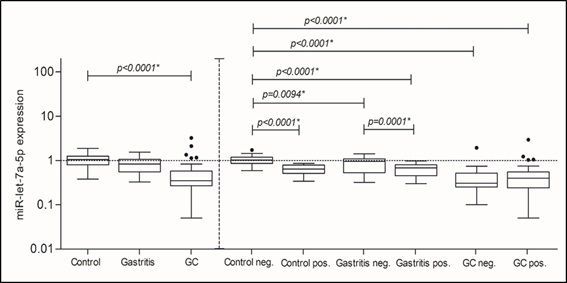
The miRNA-155-5p expression increased mainly in the groups: Control (median RQ: 2.170) and Gastritis (median RQ: 2.780) as well as in the Cancer group (median RQ: 1.640) colonized by H. pylori, in relation to the Control negative group (median RQ: 0.9500). This increase in expression is probably influenced by the presence of H. pylori, since this increase occurs even in the Control positive group. The same analyzes were performed for miRNA-let-7a-5p. However, when H. pylori was not considered, different results were observed for this miRNA. Statistically significant differences were found in the comparison among the three groups. (p=0<0.0001*) (Figure 2). In this case, its expression decreased in the Gastritis group (median RQ: 0.8300) and the Cancer group (median RQ: 0.3500) compared to the Control group (median RQ: 1.050). In subsequent analyzes, similar results were found. When we compared the Control negative group with: Control positive, Gastritis negative and positive, and with GC negative and positive groups and the same in association: Gastritis positive and negative groups (Figure 2).
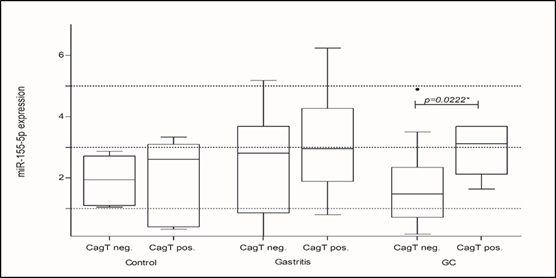
In this case, the significant decrease of miRNA-let-7a-5p expression in Gastritis and GC groups positive for H. pylori, in agreement with previous studies, highlighted its decrease in H. pylori induced inflammatory processes [17,18,24]. Another analysis was performed aiming to evaluate a possible association between cagA and cagT virulence factors from H. pylori with changes in the miRNA-155-5p and miRNA-let-7a-5p expression. Both virulence factors were compared (cagA neg. vs cagA pos. and cagT neg. vs cagT pos.) for the expression of both miRNAs in each studied group. However, only the presence of the cagT gene seems to influence the expression levels of miRNA-155-5p in patients with GC (p=0.0222*; Figure 3).
Discussion
GC has been described as a public health problem in the world and, therefore, has gained increasing attention [25]. Although its etiology is multifactorial, miRNAs, which are known mainly for regulating gene expression, have also stood out. Due to this ability and their role in physiological and pathological processes, their dysregulation can affect multiple signaling pathways through target genes, contributing to the development of inflammatory diseases and cancer. Some studies also suggest that H. pylori infection may influence dysregulation of specific miRNAs [11,12,23,26]. H. pylori has been linked to gastric diseases since its discovery approximately 30 years ago. Its colonization in the gastric epithelium promotes an intense inflammatory process, which is known as the key to the early stage of GC [23,27].
miRNA-155-5p originates from a conserved region of the MIR155HG gene located in the 21q21.3 region of chromosome 21. Characterized as an important regulatory factor in normal immune response and inflammatory process, it appears to act in regulating responses to a vast network of stimuli. Studies on miRNA-155-5p deficient mouses have found that they are immunodeficient, suggesting that this miRNA is necessary for the development of B and T cells and dendritic cells, which are indispensable for an effective immune response [28-31].
In this study, when the presence of the bacterium was considered in the analyzes, we found an increase of miRNA-155- 5p expression in groups positive for H. pylori, suggesting that the bacterium is inducing this increase, since it occurs even in the Control positive group. Although no statistically significant differences were found disregarding the presence of the bacteria, we found an increase in its expression in the Gastritis and Gastric Cancer groups in relation to the Control group. Similar to our results, Mahesh and Biswas (2019) [14] found increased miRNA-155-5p expression in gastric cancer cells by signaling molecules that induce inflammation. In contrast, Ma et al. (2016) [29] observed reduced expression in the Gastric Cancer group, suggesting an anti-tumor effect of miRNA-155- 5p. In addition, our results suggest that the cagT gene expression promotes increased expression of this miRNA in patients with GC. However, the relation between the virulence factors and miRNAs is still poorly described, and we believe that other analyzes could promote a better understanding of the relationship between cagT gene and the miRNA-155-5p. At the same time, some studies have found that cagT is more expressed in patients with peptic ulceration and GC, making it an important prognostic marker. Therefore, H. pylori strains that express cagT trend to develop more critical symptoms due to the intense inflammatory process [32,33].
Studies suggests that miRNA-155-5p expression may be regulated by the presence of H. pylori, although the NF-kB and AP-1 pathways are required for its expression in response to the pathogen. They also suggest that this miRNA may negatively modulate the release of some proinflammatory cytokines, such as IL-8 and GRO-a. Pathways such as IKK and FADD have been revealed as potential targets of miRNA-155 for the development of its mechanisms of action. The ability of miRNA-155- 5p to control these pathways could interfere with activation of the NF-kB pathway, associated to the inflammatory process and regulation of immune genes, which consequently would decrease inflammation in response to H. pylori infection. However, despite the activation of these mechanisms to perform their action, the H. pylori colonization and secretion of virulence factors, specially cagT, continues to stimulate an inflammatory response, which appears as a cycle [10,14,31,34,35]. Some studies have also shown that miRNA-155-5p is usually overexpressed in solid tumors, suggesting an oncogenic action of this miRNA; however, this profile depends on tissue and cell type [14,36], although its exact role in gastric cancer is still not well understood.
miRNA-let-7a-5p was first identified as a gene capable of promoting the transition from the larval to adult state in Caenorhabditis elegans and acts as a new type of miRNA. it is characterized as a tumor suppressor in different types of tumors. Its action is through several signaling pathways, such as PKM2, associate to proliferation, invasion, and apoptosis in some types of cancer. Some researchers have shown that miRNA-let-7a expression levels are generally low in cancer patients [16,18].
Here, when the presence of H. pylori was not taken into account, we found a statistically significant difference among the three groups studied, as previously mentioned. When we considered the presence of H. pylori, we demonstrated a decrease in patients with gastritis and cancer, especially in positive groups. In line with our results, Tang et al. (2016) [17] also demonstrated decreased miR-let-7a expression in CG patients, and that this loss was associated with increased tumor cell proliferation, invasion, and worse disease prognosis. Similar to the other miRNA studied here, miRNA-let-7a-5p also appears to have its expression regulated by the presence of H. pylori through the NF-kB pathway, along with the MAPK pathway. H. pylori virulence factors activate the NF-kB pathway, suggesting that the presence of H. pylori promotes continuous activation of NFkB, intensifying the inflammatory process and influencing in miRNA-let-7a-5p expression by activating these pathways [18]. Johnson et al. (2007) [37] found a high expression of miRNA-let7a-5p in healthy cells, as well as its ability to directly regulate some stages of the cell cycle, such as RAS and CDK6, and thus control cell proliferation. Two further hypotheses are suggested by them that the alteration of miRNA is a consequence of the disease or that this miRNA is itself contributing to the development of the tumor.
Taken together, our results demonstrated an increased expression of miRNA-155-5p and a decreased in miRNA-let-7a-5p expression in patients with Gastritis and GC, both of which appear to be influenced by the presence of H. pylori. Moreover, cagT gene of H. pylori promoted an increased miRNA-155-5p expression in GC group. Therefore, due to the importance of these miRNAs in cancer development, both could become future therapeutic targets and contribute to the development of biomarkers for early detection of Gastric Cancer.
Declarations
Conflicts of Interest: No conflicts of interest to declare.
Acknowledgments: The authors are grateful for the financial support of the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), grant numbers: 2018/08481-1 and 2018/02008-2.
Data availability statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Marginean MO, Marginean CO, Melit LE, Voidazan S, Moldovan V, Banescu C. The impact of host’s genetic susceptibility on Helicobacter pylori infection in children. Medicine. 2017; 96: e7612.
- Rasmussen LT, de Labio RW, Gatti LL, da Silva LC, de Queiroz VF, et al. Helicobacter pylori detection in gastric biopsies, saliva and dental plaque of Brazilian dyspeptic patients. Mem Inst Oswaldo Cruz. 2010; 105: 326-330.
- Melchiades JL, Zabaglia LM, Sallas ML, Orcini WA, Chen E, Smith MAC, et al. Polymorphisms and haplotypes of the interleukin 2 gene are associated with an increased risk of gastric cancer. The possible involvement of Helicobacter pylori. Cytokine. 2017; 96: 203-207.
- Sabbagh P, Javanian M, Koppolu V, Vasigala VKR, Ebrahimpour S. Helicobacter pylori infection in children: an overview of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2019; 38: 1035-1045.
- Hussein NR. Helicobacter pylori and gastric cancer in the Middle East: A new enigma? World J Gastroenterol. 2010; 16: 3226- 3234.
- Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. NIH. 2013; 12: 203-213.
- Ishimoto T, Baba H, Izumi D, Sugihara H, Kurashige J, Iwatsuki M, et al. Current perspectives toward the identification of key players in gastric cancer microRNA dysregulation. Int J Cancer. 2016; 138: 1337-1349.
- Kiselev FL. MicroRNA and cancer. Mol Biol. 2014; 48: 232-242.
- Molina-Castro S, Pereira-Marques J, Figueiredo C, Machado JC, Varon C. Gastric cancer: Basic aspects. Helicobacter. 2017; 22: 1-7.
- Zabaglia LM, Sallas ML, Santos MP Dos, Orcini WA, Peruquetti RL, Constantino DH, et al. Expression of miRNA-146a, miRNA-155, IL-2, and TNF-α in inflammatory response to Helicobacter pylori infection associated with cancer progression. Ann Hum Genet. 2018; 82: 135-142.
- Noto JM, Peek RM. The role of microRNAs in Helicobacter pylori pathogenesis and gastric carcinogenesis. Front Cell Infect Microbiol. 2011; 1: 21.
- Rossi AFT, Cadamuro ACT, Biselli-Périco JM, Leite KRM, Severino FE, Reis PP, et al. Interaction between inflammatory mediators and miRNAs in Helicobacter pylori infection. Cell Microbiol. 2016; 18: 1444-1458.
- Li S, Zhang T, Zhou X, Du Z, Chen F, Luo J, et al. The tumor suppressor role of mir-155-5p in gastric cancer. Oncol Lett. 2018; 16: 2709-2714.
- Mahesh G, Biswas R. MicroRNA-155: A Master Regulator of Inflammation. J Interf Cytokine Res. 2019; 39: 321-330.
- Wan J, Xia L, Xu W, Lu N. Expression and function of miR-155 in diseases of the gastrointestinal tract. Int J Mol Sci. 2016; 17: 1-11.
- Guo M, Zhao X, Yuan X, Jiang J, Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017; 8: 28226-28236.
- Tang R, Yang C, Ma X, Wang Y, Luo D, Huang C, et al. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in gastric cancer. Oncotarget. 2016; 7: 5972-5984.
- Matsushima K, Isomoto H, Inoue N, Nakayama T, Hayashi T, Nakayama M, et al. MicroRNA signatures in Helicobacter pyloriinfected gastric mucosa. Int J Cancer. 2011; 128: 361-370.
- Stolte M, Meining A. The updated Sydney system: Classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001; 15: 591-598.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001; 25: 402-408.
- dos Santos MP, Sallas ML, Zapparoli D, Orcini WA, Chen E, Smith M de AC, et al. Lack of Association between IL-6 Polymorphisms and Haplotypes with Gastric Cancer. J Cell Biochem. 2019; 120: 9448-9454.
- Sallas ML, Melchiades JL, Zabaglia LM, Moreno JR do P, Orcini WA, Chen ES, et al. Prevalence of Helicobacter pylori vacA, cagA, dupA and oipA Genotypes in Patients with Gastric Disease. Adv Microbiol. 2017; 07: 1-9.
- Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014; 345: 196-202.
- Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao L, et al. Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget. 2018; 9: 12351-12364.
- Wang Q, Liu G, Hu C. Molecular classification of Gastric Adenocarcinoma. Gastroenterol Res. 2019; 12: 275-282.
- Shimizu T, Marusawa H, Watanabe N, Chiba T. Molecular Pathogenesis of Helicobacter pylori-Related Gastric Cancer. Gastroenterol Clin North Am. 2015; 44: 625-38.
- Payão SLM. Helicobacter pylori and its reservoirs: A correlation with the gastric infection. World J Gastrointest Pharmacol Ther. 2016; 7: 126.
- Belair C, Darfeuille F, Staedel C. Helicobacter pylori and gastric cancer: Possible role of microRNAs in this intimate relationship. Clin Microbiol Infect. 2009; 15: 806-812.
- Ma Z, Ma Y, Xia Q, Li Y, Li R, Chang W, et al. MicroRNA-155 expression inversely correlates with pathologic stage of gastric cancer and it inhibits gastric cancer cell growth by targeting cyclin D1. J Cancer Res Clin Oncol. 2016; 142: 1201-1212.
- Tili E, Michaille J-J, Cimino A, Costinean S, Dumitru CD, Abdullah M, et al. Modulation of miR-155 and miR-125b Levels following Lipopolysaccharide/TNF-α Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J Immunol. 2007; 179: 5082-5089.
- Xiao B, Liu Z, Li B, Tang B, Li W, Guo G, et al. Induction of microRNA‐155 during Helicobacter pylori Infection and Its Negative Regulatory Role in the Inflammatory Response . J Infect Dis. 2009; 200: 916-925.
- Mattar R, Monteiro MS, Marques SB, Zilberstein B, Hashimoto CL, Carrilho FJ. Association of LEC and tnpA Helicobacter pylori genes with gastric cancer in a Brazilian population. Infect Agent Cancer. 2010; 5: 2-4.
- Pacheco AR, Proença-Módena JL, Sales AIL, Fukuhara Y, Da Silveira WD, Pimenta-Módena JL, et al. Involvement of the Helicobacter pylori plasticity region and cag pathogenicity island genes in the development of gastroduodenal diseases. Eur J Clin Microbiol Infect Dis. 2008; 27: 1053–1059.
- Ma X, Buscaglia LEB, Barker JR, Li Y. MicroRNAs in NF- k B signaling. J Mol Cell Biol. 2011; 0: 159-166.
- Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 Is Essential for the T Cell-Mediated Control of Helicobacter pylori Infection and for the Induction of Chronic Gastritis and Colitis . J Immunol. 2011; 187: 3578-3586.
- Bayraktar R, Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018; 37: 33-44.
- Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007; 67: 7713-7722.
- Scholte GH, van Doorn LJ, Quint WG, Lindeman J. Polymerase chain reaction for the detection of Helicobacter pylori in formaldehyde-sublimate fixed, paraffin-embedded gastric biopsies. Diagn Mol Pathol. 1997; 6: 238-243.
- Yakoob J, Fatima SS, Abbas Z, Mustafa SF, Khan HAR, Raghib MF, et al. Distribution of gastric carcinoma in an area with a high prevalence of Helicobacter pylori. Turkish J Gastroenterol. 2017; 28: 98-103.