Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Role of cystatin C in evaluation of renal disease in patients with cirrhosis
Mohamad-Sherif Mogawer1; Sahar Abdel-Rahman Nassef2; Samah Mohamad Abd-Elhamid3; Shaimaa Elkholy4; Nahla Emad Abd-ElAziz5; Ula Mabid Al-Jarhi6; Abeer Awad Abdellatif7*
1 Professor of Hepatology and Internal Medicine Department, Faculty of Medicine, Cairo University, Egypt.
2 Professor of Vascular and Internal Medicine Department, Faculty of Medicine, Cairo University, Egypt.
3 Assistant Professor of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Egypt.
4 Assistant Professor of Internal Medicine Department, Faculty of Medicine, Cairo University, Egypt.
5 Lecturer, Internal Medicine Department, Faculty of Medicine, Cairo University, Egypt
6 Assistant Professor, Internal Medicine Faculty of Medicine, Cairo University, Egypt.
7 Lecturer, Internal Medicine Department, Faculty of Medicine, Cairo University, Egypt.
*Corresponding Author: Abdellatif AA
Lecturer, Internal Medicine Department, Faculty of
Medicine, Cairo University, Egypt.
Email: beero4a@yahoo.com
Received: Dec 24, 2021
Accepted: Feb 17, 2022
Published: Feb 24, 2022
Archived: www.jjgastro.com
Copyright: © Abdellatif AA (2022).
Abstract
Background: Renal dysfunction is not a rare entity cirrhotic patients and if occurs usually associated with poor outcome. Currently, there are limitations in assessing renal function in cirrhotic patients with lacking markers for early diagnosis and identification of high-risk patients.
Aim: To evaluate the role of serum cystatin C in diagnosis and early prediction of renal affection in cirrhotic patients and hepatorenal syndrome.
Methods: This study included 85 subjects (50 patients and 35 healthy controls). Clinical assessment, liver function tests, hepatitis C virus antibody, kidney function tests, serum cystatin C, abdominal ultrasound were done on all subjects.
Results: Univariate regression analysis for hepatorenal syndrome showed a statistically significant positive correlation with Model For End-Stage Liver Disease Score (p-value <0.0001). On the contrary, our study has demonstrated that serum Cystatin C can be proposed as a significant marker for advanced liver disease. However, it could not differentiate hepatorenal syndrome from Child C cirrhotic patients (pvalue <0.05), so it cannot be used for early prediction of hepatorenal syndrome.
Conclusion: Serum Cystatin C is a significant marker for advanced liver disease. However, it could not be a good predictor of hepatorenal syndrome.
Keywords: liver cirrhosis; serum cystatin C; hepatorenal syndrome.
Abbreviations: HRS: Hepatorenal Syndrome; AKI: Acute Kidney Injury; GFR: Glomerular Filtration Rate; RAAS: Renin-Angiotensin-Aldosterone System; MELD: Model for End-Stage Liver Disease; CLCr: Creatinine Clearance; ELISA: Enzyme-linked Immunosorbent Assay; US: Ultrasound; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C Virus.
Citation: Mogawer MS, Nassef SA, Abd-Elhamid SM, Elkholy S, Abdellatif AA, et al. Role of cystatin C in evaluation of renal disease in patients with cirrhosis. Japanese J Gastroenterol Res. 2022; 2(3): 1062.
Introduction
Renal dysfunction is a frequently seen complication in cirrhotic patients that could not be directly related to the cause of cirrhosis as ischemia, sepsis, drugs or it raises from causes directly related to cirrhosis such as nonalcoholic fatty liver disease, alcohol, hepatitis B, and C related glomerulopathy, also it can occur as a consequence of cirrhosis and portal hypertension in what is called hepatorenal syndrome (HRS) [1]. HRS is a functional form of acute kidney injury (AKI) that commonly occurs as a serious complication of cirrhosis and is associated with high morbidity and mortality rates [2,3].
Pathogenesis of renal affection in cirrhotic patients could be due to circulatory disturbances secondary to decompensated cirrhosis (i.e., hepatorenal syndrome) resulting from intra-renal vasoconstriction that compromises glomerular filtration rate with secondary retention of sodium and water; [4] also peripheral vasodilatation that occurs in advanced liver disease triggers a myriad of compensatory hormonal and neurohormonal vasoconstrictors which reduce effective renal blood flow,[5] or it could occur independently of hemodynamic disturbances of cirrhosis (i.e., volume depletion, drug-induced nephrotoxicity, glomerulopathies) [6].
Still, renal imaging and histology are the cornerstones for the evaluation of renal affection [7]. Progression of AKI in cirrhotic patients carries poor outcomes whether prerenal AKI (hypovolemia and HRS-AKI) or intrinsic AKI (mainly acute tubular necrosis) [8]. Unfortunately, routine renal function tests could not provide a measure for actual GFR in hepatic patients [9]. There are major limitations to creatinine estimation in chronic liver disease patients as it is production reduced in those patients either because of reduced muscle mass or reduced protein intake that results in false low level with subsequent falsely “low” estimated GFR. Also, several factors as liver failure, malnutrition, aging, and decreasing muscle mass affect serum level of creatinine [10]. In this context, serum creatinine considers an insensitive marker especially for early acute changes in kidney function [6]. Cystatin C is a non-glycosylated 13 kDa protein, produced at a constant rate by all nucleated cells and can be filtered freely in the glomeruli, reabsorbed, and metabolized in the proximal tubules. [11] It is not affected by race, age, muscle mass, or liver function therefore less biased than sCr-based formulas and has higher performance in cases of lower GFR [12, 13, 14]. Accordingly, the main aim of this work was to evaluate the role of serum cystatin C as possible markers in the diagnosis and prediction of renal affection in patients with liver cirrhosis.
Materials and methods
Subjects
This prospective observational study enrolled 85 subjects (50 patients and 35 healthy controls), aged 18 years or above, with Child C liver cirrhosis (the diagnosis of cirrhosis was based on a combination of clinical, laboratory, and ultrasonographic findings) with documented normal baseline serum creatinine within 1 year less than 1.5 mg/dL. The patients were randomly selected among a group of patients presenting to the emergency room unit and admitted to the department of internal medicine in KasrAlAiny hospital during the period from January 2019 to July 2020. Patients exclud ed from the study were those with confirmed pregnancy, prior kidney or liver transplant, known hypertensive patients, or other known causes of renal insufficiency such as advanced chronic kidney disease (CKD): baseline creatinine >4.0 mg/Dl, acute or chronic renal replacement therapy, diabetic nephropathy, glomerulonephritis, urinary tract obstruction, and urinary tract infection.
Methods
Patients with liver cirrhosis were randomly selected irrespective of the presenting symptom, hepatic status, and presence of complications. All these patients were subjected to thorough history taking and clinical examination including age, gender, comorbid diseases (Diabetes, hypertension, and dyslipidemia), concomitant medications (renin-angiotensin-aldosterone system (RAAS) blockade and diuretics), symptoms, and signs of hepatic decompensation. Child-Pugh score and Model for EndStage Liver Disease (MELD) score were calculated for all patients.
Laboratory investigations
Patients’ preparation: diuretics were stopped in all patients, at least 24 h before laboratory testing. Patients were advised to adopt a low sodium diet (less than 40 mmol/d). A fresh 10 ml blood sample was collected daily. Laboratory tests were performed in Kasr Al Ainy chemical Pathology Central Lab and included: Liver function tests, HCV antibody, and Kidney function tests. Creatinine was measured from samples collected as part of routine clinical care in our institution in patients. Laboratory measurements were performed by personnel blinded to patient information.
Creatinine clearance (CLCr) was calculated by Cr Cl Cockroft Gault equation CCr= {((l 40–age) X weight)/(72xSCr)}X 0.85 (if female). eGFR calculated by MDRD formula [15]. Special lab investigation in this study includes serum Cystatin C. It was determined by the ELISA method (enzyme-linked immunosorbent assay), (Chinese kits). Serum samples were allowed to clot in a serum separator tube (about 4 hours) at room temperature. Centrifuged at approximately 1000 X g for 15 min. Analyzed immediately or stored at -20°C. Samples were diluted using the provided diluent buffer. The standard curve was plotted as the relative O.D.450 of each standard solution (Y) against the respective concentration of the standard solution (X). The human Cystatin C concentration of the samples was interpolated from the standard curve.
Abdominal ultrasound (US) was done using (IU 22, Philips machine). A convex probe (C5-1 Hz) was used and the US was done to all patients to confirm the presence of cirrhosis, to assess portal vein, hepatic artery and veins diameter, presence or absence of portal vein thrombosis, splenic size, to confirm the presence or absence of ascites and its degree, to confirm presence of focal lesions of liver and spleen and to assess kidney size, volume, and echogenicity
Statistical methodology
The analysis of our data was performed using IBM computer exploiting SPSS (statistical program for social science version 12) as follows: Description of quantitative variables as mean, SD and range. Description of qualitative variables was done as number and percentage. The Chi-square test was used to compare qualitative variables between groups. Unpaired t-test was used to compare quantitative variables, in parametric data (SD<50% mean). Mann Whitney Willcoxon U test was used in non-parametric data instead of unpaired t-test. One-way ANOVA (analysis of variance) was used to compare more than two groups as regard quantitative variables. Kruskal Wallis test was used instead of ANOVA test in non-parametric data SD>50% mean. Spearman Correlation test was used to rank variables versus each other positively or inversely. Logistic regression analysis was used to find out the significant independent predictors of the dependent variable using the backward likelihood ratio technique. In this context, p-value >0.05 was considered as insignificant, p<0.05 as significant and p<0.01 as highly significant.
Ethical consideration
The study was approved by the institutional ethical committee and form review board of Kasr AlAiny hospital. Oral and written informed consents were obtained from all subjects or their eligible relatives. The medical record profession has its code of ethics which applies to all medical record practitioners. Confidentiality of data, safe data storage, and privacy rights are respected by all who handle patient information. Data was coded and patient names or identities was not appearing in any of the data collection forms or during statistical analysis.
Results
The study cohort included 27 males (54%) and 23 females (46%), their age ranged from 41–86 years (mean = 59.72), Child score ranged from 10–14, MELD score ranges from 11-38. The baseline characteristics and demographic and clinical data of the enrolled patients are presented in (Table 1). There was no significant difference in gender distribution between the patients and the control.
Table 1A: Gender distribution among both patients and control groups
Gender |
Patients |
Control |
Number (%) |
Number (%) |
|
Male |
27 (54%) |
20 (57.1%) |
Female |
23 (46%) |
15 (42.9%) |
Total |
50 (100%) |
35 (100%) |
Table 1B: Age distribution among both patients and control groups.
Age |
Mean |
SD |
95% Confidence Interval for Mean |
|
Lower Bound |
Upper Bound |
|||
Control |
54.74 |
11.1 |
50.9 |
58.6 |
Patients |
59.72 |
8.1 |
57.4 |
62.02 |
Table 1C: Basic laboratory data of the patients.
Variables |
Mean (SD)/ Median (IQR)* |
HB (g/dl) |
9.61 (1.7) |
MCV (fl) |
87.05 (8.9) |
MCH (pg) |
28.8 (3.1) |
TLC |
6 (5.09)* |
PLT |
91 (83)8* |
PT (sec) |
20.2 (6.5)* |
PC (%) |
48.3 (15.05) |
ALT(U/l) |
30 (27.5)* |
AST(U/l) |
49 (38)* |
ALB(g/dl) |
2.13 (0.5) |
TP(g/dl) |
6.45 (0.65) |
BILI T(mg/dl) |
2.2 (2.7)* |
BILI D(mg/dl) |
0.98 (1.2)* |
GGT (U/L) |
26 (36)* |
ALP (U/L) |
100 (88)* |
LDH (U/L) |
320 (303)* |
CA (mg/dl) |
7.69 (0.7) |
PO (mg/dl) |
3.41 (0.9) |
Mg (mg/dl) |
1.96 (0.3) |
UA (mg/dl) |
7.5 (3.9)* |
*IQR: interquartile range, HB:hemoglobin, TLC:total leucocytic count, PLT:platelet count, MCV:mean corpuscular volume, MCH:mean corpuscular hemoglobin, PT:prothrombin time, PC:prothrombin concentration, ALT:alanine transaminase,AST:aspartate transaminase, ALB:albumin, TP:total proteins, BILI T:bilirubin total, BILI D:bilirubin direct, GGT:gamma glutamyl transferase, ALP:alkaline phosphatase, LDH:lactic acid dehydrogenase, CA:calcium, PO:phosphorus, Mg:magnesium, UA:uric acid.
Table 1D: Basic laboratory data of the controls.
Variables |
Mean |
SD |
HB (g/dl) |
12.9 |
1.2 |
MCV (fl) |
83.8 |
7.4 |
MCH (pg) |
30.3 |
2.7 |
TLC |
5.35 |
1.5 |
PLT |
252.5 |
68 |
PT (sec) |
13.12 |
0.56 |
PC (%) |
97 |
8 |
ALT(U/l) |
20.15 |
9.5 |
AST(U/l) |
24.9 |
9.3 |
ALB(g/dl) |
4.14 |
0.3 |
TP(g/dl) |
6.9 |
0.4 |
BILI T(mg/dl) |
0.75 |
0.2 |
BILI D(mg/dl) |
0.3 |
0.26 |
GGT (U/L) |
17.4 |
8 |
ALP (U/L) |
90.1 |
21 |
LDH (U/L) |
173.42 |
67 |
CA (mg/dl) |
8.9 |
0.4 |
PO (mg/dl) |
3.1 |
0.3 |
Mg (mg/dl) |
1.8 |
0.2 |
UA (mg/dl) |
4.1 |
0.7 |
HB:hemoglobin, TLC:total leucocytic count, PLT:platelet count, MCV:mean corpuscular volume, MCH:mean corpuscular hemoglobin, PT:prothrombin time, PC:prothrombin concentration, ALT:alanine transaminase,AST:aspartate transaminase, ALB:albumin, TP:total proteins, BILI T:bilirubin total, BILI D:bilirubin direct, GGT:gamma glutamyl transferase, ALP:alkaline phosphatase, LDH:lactic acid dehydrogenase, CA:calcium, PO:phosphorus, Mg:magnesium, UA:uric acid.
Table 1E: Child-Turcott-Pugh and MELD scores of the patients.
Variables |
Median |
IQR |
Child score |
10 |
2 |
MELD |
19 |
7 |
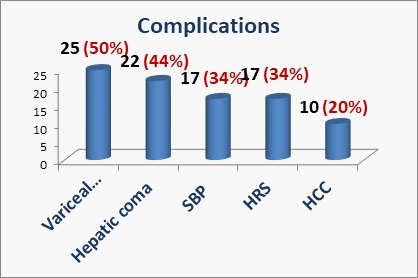
The frequency of various presenting complications of liver cirrhosis was higher for variceal bleeding (50%) followed by hepatic coma (44%), SBP (34%), HRS (34%), and HCC (20%) as demonstrated in (Figure 1). Most of the patients had ascites in 46 patients (92%) and only 6 patients (12%) had portal vein thrombosis as reported by abdominal ultrasound.
As expected, our study showed a statistically significant difference in basic laboratory data of patients and control groups as regards Hb, MCH, PLT, PT, PC, ALT, AST, Albumin, total proteins, bilirubin (total and direct), GGT, LDH, calcium and uric acid where patients were statistically higher for alanine transaminase, aspartate transaminase, total proteins, total bilirubin, direct bilirubin, gamma-glutamyl transferase, lactic acid dehydrogenase and lower for hemoglobin level, MCV, platelet count, prothrombin concentration and albumin (p<0.0001). On the other hand, there was no significant difference as regard MCV, TLC, ALP, PO, and Mg.
The total number of patients who completed the study till the end was (48) patients while (2) female patients dropped out due to mortality. Both patients presented with HRS and hepatic coma, with Child scores 11 and 14, MELD 29 and 38, respectively. Kidney function tests of the patients in the study cohort are shown in (Table 2). The serum Cystatin C level was higher among patients (mean ±SD 4.31 ± 1.04 respectively) than the control group (mean and SD equal 1.15, 0.62 respectively) using t-test (Table 3).
Table 2A: Kidney function tests of the included patients.
Variables |
Median |
IQR |
Serum creatinine (mg/dl) |
1.28 |
0.85 |
Blood urea (mg/dl) |
57.9 |
50.5 |
Serum Na (mEq/L) |
136 |
11 |
Serum K (mEq/L) |
4 |
1 |
eGFR (ml/min/1.73m2) |
56.01 |
44.8 |
Cr Clearance (ml/min) |
58.76 |
37.13 |
Na: Sodium, K: Potassium, EGFR: Estimated Glomerular Filteration Rate, Cr Clearance: Creatinine C learance.
Table 2B: Kidney function tests of the control group.
Variables |
Mean |
SD |
Creatinine (mg/dl) |
0.61 |
0.19 |
Urea (mg/dl) |
27.9 |
9.72 |
NA (mEq/L) |
139.17 |
2.48 |
K (mEq/L) |
4.04 |
0.32 |
Table 3: Comparing Cystatin C of the both patients and control groups.
CystatinC (mg/dl) |
Mean |
SD |
t |
P-value |
Control |
1.15 |
0.62 |
-16.02 |
<0.0001 |
Patients |
4.31 |
1.04 |
-17.5 |
P value is considered significant when it’s value < 0.05.
There was a positive correlation between cystatin C on one side and each of age, ALT, AST, TP, total bilirubin, direct bilirubin, GGT, ALP, LDH, serum creatinine, blood urea, and uric acid on the other. Also, cystatin C showed a negative correlation with each of hemoglobin, MCH, platelet count, PC, albumin, serum sodium, and calcium using Spearman’s rho (Table 4). There was a statistically significant positive correlation between serum Cystatin C and ultrasound variables including cortical thickness. As regards ascites degree, it had a significant negative correlation (Table 4).
Table 4: Correlation between serum Cystatin C, laboratory and ultrasound variables.
Cystatin C |
Correlation oefficient |
P-value |
Age (years) |
0.21 |
0.046 |
HB (g/dl) |
-0.63 |
<0.0001 |
MCH (pg) |
-0.32 |
0.003 |
Platelets (10^3/Cmm ) |
-0.675 |
<0.0001 |
PC (%) |
-0.7 |
<0.0001 |
ALT(U/l) |
0.425 |
<0.0001 |
AST(U/l) |
0.484 |
<0.0001 |
TP(g/dl) |
0.465 |
<0.0001 |
Albumin (g/dl) |
-0.75 |
<0.0001 |
Total bilirubin (mg/dl) |
0.546 |
<0.0001 |
Direct bilirubin(mg/dl) |
0.465 |
<0.0001 |
GGT(U/l) |
0.301 |
0.005 |
LDH(U/l) |
0.548 |
<0.0001 |
Serum Creatinine(mg/dl) |
0.67 |
<0.0001 |
Blood urea(mg/dl) |
0.595 |
<0.0001 |
Serum Na(mEq/L) |
-0.26 |
0.014 |
Calcium(mg/dl) |
-0.6 |
<0.0001 |
Serum uric acid(mg/dl) |
0.62 |
<0.0001 |
Ascites degree |
0.415 |
0.003 |
Kidney LongD (mm) |
-0.120 |
0.407 |
Kidney TransD (mm) |
-0.194 |
0.176 |
KidneyCortical thickness(mm) |
-0.393 |
0.005 |
Kidney Volume (ml) |
-0.217 |
0.13 |
PSV(O) cm/s |
-0.205 |
0.06 |
PSV (H) cm/s |
-0.017 |
0.88 |
RI (H) |
0.702 |
<0.0001 |
RI (Interlobar artery) |
0.713 |
<0.0001 |
Mortality |
0.036 |
0.741 |
There was no statistically significant difference in the ultrasonographic data of patients as regards kidney diameters, volume, and echogenicity. All were within the normal range. In our study, there was a statistically significant difference in subgroups of the patients as regard MELD score by using KruskalWallis Test. On the other hand, there is no significant difference as regard other variables (Table 5). Also, there was a positive correlation between Mortality, MELD, D BILI, GGT, creatinine, urea, Na, and negative correlation with other variables using Spearman’s rho test (Table 6).
Table 5: Comparing the data of the subgroups of the patients.
|
Chi-Square |
df |
P-value |
Gender |
3.973 |
2 |
0.137 |
Age |
.856 |
2 |
0.652 |
Child score |
1.976 |
2 |
0.372 |
MELD |
9.095 |
2 |
0.011 |
variceal bleeding |
4.261 |
2 |
0.119 |
Hepatic coma |
4.040 |
2 |
0.133 |
SBP |
4.853 |
2 |
0.088 |
HCC |
2.397 |
2 |
0.302 |
P value is considered significant when it’s value < 0.05.
Table 6: Correlation between Mortality and other variables.
Mortality |
Correlation coefficient |
P-value |
MELD |
0.326 |
0.021 |
PC (%) |
-0.232 |
0.033 |
BILD (mg/dl) |
0.22 |
0.042 |
GGT (U/l) |
0.219 |
0.045 |
CREAT (mg/dl) |
0212 |
0.051 |
UREA (mg/dl) |
0.283 |
0.046 |
Na (mEq/L) |
0.225 |
0.038 |
eGFR(ml/min/1.73m2) |
-0.29 |
0.041 |
Cr clearance(ml/min) |
-0.276 |
0.053 |
K long D (mm) |
-0.318 |
0.024 |
P value is considered significant when it’s value < 0.05.
By using Univariate regression analysis, there was a statistically significant difference between HRS and MELD. On the other hand, there is no significant difference as regard other variables (Table 7). Multivariable regression analysis proved that Cystatin C was non-significant in this analysis. When comparing HRS with a non-HRS group we found that there was a statistically significant difference as regards MELD, and cystatin C.
Table 7: Univariate regression analysis for hepatorenal syndrome.
HRS |
Coefficient |
Odd's ratio |
95% CI |
P-value |
MELD |
0.328 |
1.38 |
1.14-1.7 |
<0.0001 |
Cystatin C (mg/dl) |
0.55 |
1.74 |
0.9-3.18 |
0.06 |
P value is considered significant when it’s value < 0.05.
Discussion
Several mechanisms contribute to the development of renal affection throughout the progressive course of cirrhosis; consequently, many cases of renal affection in patients with cirrhosis seem to have a multifactorial etiology. Still, no gold standard test exists for early diagnosis and prediction of renal impairment in cirrhotic patients [8].
So, our study aimed to evaluate the role of serum cystatin C as possible markers in the diagnosis and prediction of renal affection in patients with liver cirrhosis. Our study included 50 patients with child C liver cirrhosis presenting to Kasr Al Ainy inpatient unit with various complications of liver cirrhosis. Their age ranged from 41–86 years, including 27 males and 23 females. The study also included 35 healthy controls.
The current study demonstrated that serum cystatin C level was higher among all patients than in the control group. This may propose that serum cystatin C could be a marker of advanced liver disease. However, serum cystatin C did not show any significant association with HRS. Woitas et al [16] had also found that cystatin C was significantly higher in Child-Pugh B and C patients when compared to Child-Pugh A patients. However, no difference was observed between patients with Child-Pugh B and C. However, Chung et al [17] found that serum Cystatin C could be proposed as a marker of liver disease stage and is a more sensitive indicator of renal function in patients with cirrhosis in comparison to serum creatinine level.
On the contrary, in our present study serum cystatin C level was not higher in hepatorenal patients than in Child C cirrhotic patients without HRS (p = 0.186) so it could’t be a good marker for detection of renal impairment in liver cirrhosis patients or a predictor for hepatorenal syndrome. Several studies suggested that serum CysC and CysC-based formulae are more useful with superior diagnostic accuracy over other markers and equations in detecting moderate and severe renal impairment in patients with liver cirrhosis [10,18,19]. A study conducted on HCV-related cirrhosis reported that the GFRcr-cys formula proved to be of best precision and accuracy in estimating GFR irrespective of sex or degree of mGFR [20]. Our study also showed that HRS has a statistically significant positive correlation with MELD by univariate regression. Therefore, we concluded that it is unreliable as a marker for detection of renal impairment in liver cirrhosis patients or a predictor for hepatorenal syndrome. From a critical point of view, this work has some limitations. The first one was the small sample of the studied patients. We also were not able to follow up on patients with suspected HRS and normal renal function.
Conclusions
We conclude that serum cystatin C could be proposed as a marker of liver disease but it is not a good marker for the prediction of renal impairment in liver cirrhosis patients.
Recommendations
Finding a good marker of the renal function is a crucial issue to patients with liver cirrhosis due to the impact of early diagnosis and prediction of the renal impairment on the prognosis of these patients.
Declarations
Ethics approval and consent to participate: The study was approved by institution ethical committee and form review board of Kasr Al Ainy hospital. Oral and written informed consents were obtained from the patient or from his eligible relatives. The ethical approval number and date is not available.
Consent for publication: Oral and written informed consents were obtained from the patient or from his eligible relatives.
Availability of data and material: Not Applicable.
Competing Interests: The authors declare no potential competing interests.
Funding: Authors received no funding for this study.
Conflicts of interests: We have no financial or other relationships that might lead to a conflict of interest.
Authors’ contributions
MS analyzed and interpreted the patients’ data and general supervision of the research group.
SA analyzed and interpreted the patients’ data.
SM participated in interpretation of the data of the patients.
SE analyzed, interpreted the patients’ data and statistical analysis.
NE participated in interpretation of the data of the patients.
UM analyzed and interpreted the data of the patients and helped in writing the manuscript.
AA was a major contributor in writing the manuscript.
All authors read and approved the final manuscript.
Acknowledgements: We would like to acknowledge our great Kasr Al Ainy Hospital, and its workers, nurses and staff members, for all the support and help in this study and throughout our careers.
References
- Lum EL, Homkrailas P, Bunnapradist S. Evaluation of Renal Disease in Patients with Cirrhosis. J Clin Gastroenterol. 2020; 54: 314-321.
- Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956; 271: 1121-1125.
- Mauro E, Garcia-Olveira L, Gadano A. End-stage liver disease: Management of hepatorenal syndrome. Liver Int. 2021; 41: 119- 127.
- Hiremath SB, Srinivas LD. Survival benefits of terlipressin and non-responder state in hepatorenal syndrome: a meta-analysis. Indian J Pharmacol. 2013; 45: 54-60.
- Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008; 28: 81-95.
- Carvão J, Carvão JN, Pereira V, Jasmins L. Challenges in measuring renal function in liver cirrhosis: are there implications in clinical practice?. Acta Gastroenterol Belg.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007; 56: 1310-1318.
- Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis [published correction appears in Nat Rev Nephrol. 2020 Jan 27]. Nat Rev Nephrol. 2020; 16: 137-155.
- McCormick PA, Donnelly C. Management of hepatorenal syndrome. Pharmacol Ther. 2008; 119: 1-6.
- Kim DJ, Kang HS, Choi HS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol. 2011; 17: 130-138.
- Gupta K, Bhurwal A, Law C, et al. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J Gastroenterol. 2021; 27: 3984-4003.
- Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003; 41: 269-278.
- Ashalatha VL, Bitla AR, Kumar VS, et al. Biomarker response to contrast administration in diabetic and nondiabetic patients following coronary angiography. Indian J Nephrol. 2017; 27: 20-27.
- Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C--a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008; 29: 47-62.
- Wang D, Feng JF, Wang AQ, Yang YW, Liu YS. Role of Cystatin C and glomerular filtration rate in diagnosis of kidney impairment in hepatic cirrhosis patients. Medicine (Baltimore). 2017; 96: e6949.
- Marottoli RA, Cooney LM, Wagner R, Doucette J, Tinetti ME. Predictors of automobile crashes and moving violations among elderly drivers. Ann Intern Med. 1994; 121: 842-846.
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001; 33: 464-470.
- E Randers, P Ivarsen, EJ Erlandsen, EF Hansen, NK Aagaard, F Bendtsen & H Vilstrup. Plasma cystatin C as a marker of renal function in patients with liver cirrhosis, Scandinavian Journal of Clinical and Laboratory Investigation. 2002; 62: 129-134.
- Omar M, Abdel-Razek W, Abo-Raia G, Assem M, El-Azab G. Evaluation of Serum Cystatin C as a Marker of Early Renal Impairment in Patients with Liver Cirrhosis. Int J Hepatol. 2015; 2015: 309042.
- El-Makarem, MAERA, Mousa MM, Ayaad LA et al. Comparative study of various glomerular filtration rate estimating equations in Egyptian patients with hepatitis C virus-related liver cirrhosis: a single-center observational study. Egypt Liver Journal 11, 23, 2021.