Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Diagnostic performance of mac-2-binding protein glycosylation isomer (m2bpgi), compared to transient elastography to assess liver stiffness in treatment naïve chronic hepatitis C patients
Andri Sanityoso Sulaiman*; Irsan Hasan; Cosmas Rinaldi A Lesmana; Juferdy Kurniawan; Chyntia Olivia Maurine Jasirwan; Saut Nababan; Kemal Fariz Kalista; Rachmadianti Sukma Hanifa; Rino Alvani Gani
Hepatobiliary Division, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Dr. Cipto Mangunkusumo National General Hospital, Jakarta, Indonesia.
*Corresponding Author : Sulaiman AS
Hepatobiliary Division, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Dr.
Cipto Mangunkusumo National General Hospital,
Jakarta, Indonesia.
Email: andri_sani@yahoo.com;
andri.sanityoso@ui.ac.id
Received : Dec 20, 2021
Accepted : Feb 07, 2022
Published : Feb 14, 2022
Archived : www.jjgastro.com
Copyright : © Sulaiman AS (2022).
Abstract
Background: Liver fibrosis is an essential factor in the management of Hepatitis C virus infection. Its assessment is crucial in the decisionmaking regarding the therapeutic decisions, and the patients’ follow up. However, the established liver measurement methods have several limitations. Therefore, the study aimed to assess the role of Mac-2-Binding Protein Glycosylation Isomer (M2BPGi) as a novel biomarker to measure liver stiffness in treatment naïve Chronic Hepatitis C Indonesian patients.
Methods: Serum M2BPGi level and Transient Elastography results were evaluated in 56 Chronic Hepatitis C patients and 48 healthy controls. Pearson correlation analysis was conducted to find the correlation between the level of M2BPGi and Transient Elastography result. ROC analysis was conducted to find the optimum cut-off to assess fibrosis’s degree among Chronic Hepatitis C Patients.
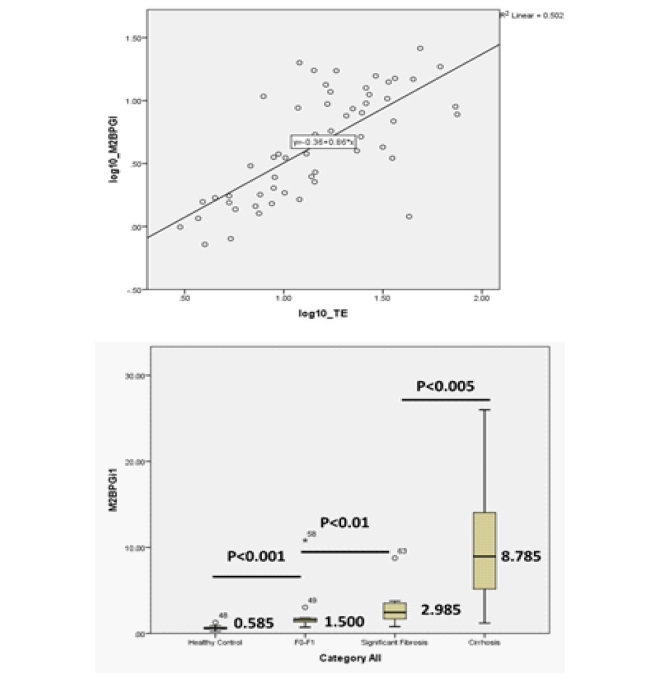
Results: The level of serum M2BPGi and Transient Elastography result was strongly correlated with the median level of serum M2BPGi. It was also significantly higher among Chronic Hepatitis C Patients than among healthy controls (r: 0.708, p<0.001; 0.590 COI vs. 4.130 COI, p<0.001). Among the Chronic Hepatitis C patients, the median serum of M2BPGi increased according to the degree of liver fibrosis: 1.500 COI (F0-F1), 2.985 COI (F2F3) and 8.785 COI (≥F4). The optimum cut-off value for diagnosing significant fibrosis (F2-F3) was 1.820 COI (AUC: 90.8%) and for diagnosing cirrhosis (≥F4) was 3.770 COI (AUC: 89.3%).
Conclusion: Serum M2BPGi was a reliable diagnostic tool for identifying liver fibrosis in Indonesian patients with Chronic Hepatitis C.
Keywords: chronic hepatitis C; mac-2-binding protein glycosylation isomer; transient elastography.
Citation: Sulaiman AS, Hasan I, Lesmana CRL, Kurniawan J, Jasirwan COM, et al. Diagnostic performance of mac-2-binding protein glycosylation isomer (m2bpgi), compared to transient elastography to assess liver stiffness in treatment naïve chronic hepatitis C patients. Japanese J Gastroenterol Res. 2022; 2(3): 1059.
Background
Chronic Hepatitis C remains a global burden of disease due to its high prevalence. It is estimated that around 177.5 million adults (2.5% of the total global population) live with HCV infection [1]. In Indonesia, the prevalence of anti-HCV is 2.5% of the total population. This data shows an increase by 0.4% from the previous survey conducted in 2007 [2]. Moreover, nearly 400 thousand people die annually from the complication of HCV infection, such as liver cirrhosis, hepatic decompensation, or hepatocellular carcinoma [3]. In clinical practice, the quantification of liver fibrosis in patients with Chronic Hepatitis C is crucial in decision making regarding the start of therapeutic regimens and the adequate follow up of the patients. According to the current clinical practice guidelines, Chronic Hepatitis C treatment is recommended when significant fibrosis is present [4]. For those detected to have liver cirrhosis, the treatment duration will be prolonged up to 6 months, and regular screening for complications and hepatocellular carcinoma surveillance must be initiated [4,5]. Moreover, the fibrosis stage is also found to be the main predictive factors of complication once the virus is eradicated. Therefore, fibrosis regression has become a new surrogate goal of Hepatitis C Virus infection therapy [6].
The gold standard for assessing the degree of liver fibrosis is a liver biopsy. However, due to its invasive method, it could lead to some potential complications. A previous study found that around 6% of complications were observed from 1806 biopsy procedures in which 75% of these patients reported moderate and severe pain, while 33% of the others underwent prolonged hospital observation or surgical intervention due to excessive bleeding [7]. Furthermore, errors in sampling and interpretation are often found in biopsy procedures. Consequently, many clinicians prefer to use non-invasive approaches [8].
Several non-invasive approaches have been used in assessing liver fibrosis in Chronic Hepatitis C patients. One of the commonly used non-invasive modalities that have been highly validated is Transient Elastography (TE). It works to quantify the mechanical responses of liver tissues through a shear-wave velocity produced by a piston. However, the generated mechanical waves diffuse in nonviscous liquids. Hence, TE could not be used in patients with ascites condition [9]. In addition, the assessment of liver fibrosis through biomarkers has also been widely used. Some methods are considered as a “direct” method since they are directly involved in the cellular matrix accumulation process. On the other hand, several biomarkers are considered an “indirect” method because they could only portray the epiphenomena associated with the fibrogenesis process [10]. One commonly used indirect biomarker for assessing liver fibrosis is AST to platelet ratio index (APRI). Its diagnostic performance has been extensively evaluated and consistently depicted more robust positive diagnostic performance when assessing advanced fibrosis and cirrhosis. However, since it is an indirect biomarker to evaluate liver fibrosis, its application will also need adjustment according to the specific condition of the patients [10].
Mac-2-binding protein glycosylation isomer (M2BPGi) was recently found to be an alternative serum for detecting liver fibrosis. Acting as a juxtracrine messenger sent by Hepatic Stellate Cells (HSCs) to Kupffer cells during liver fibrosis enables it to depict the liver fibrosis progression directly [11]. Previous studies have confirmed that M2BPGi was useful to measure liver fibrosis in several conditions, such as Hepatitis B or C infection, autoimmune hepatitis, biliary atresia, primary biliary cirrhosis, and Non-Alcoholic Fatty Liver (NAFLD) [12-16]. Furthermore, a meta-analysis study also found that M2BPGi could be a reliable predictor for determining the stage of liver fibrosis [12].
However, another study also found that the value of M2BPGi might be different according to the etiology of the liver fibrosis, even when the stage of liver fibrosis is similar. For instance, the level of M2BPGi among Nonalcoholic steatohepatitis (NASH) tends to be lower than those in Chronic Hepatitis C or B infection with the same degree of liver fibrosis [13]. Given this uniqueness and the lack of research on the role of serum M2BPGi in assessing liver fibrosis in treatment naïve Chronic Hepatitis C patients in the Indonesian population, this study aims to find the correlation of M2BPGi level with the fibrosis measurement by using Transient Elastography, measure the cut-off point to assess significant fibrosis and cirrhosis, and evaluate its diagnostic performance on Hepatitis C Chronic patients in these population.
Methods
Patients
56 treatment naïve Chronic Hepatitis C patients in one of the National General Hospital in Jakarta were recruited for the study. Exclusion criteria were: (i) diagnosed with Hepatitis B, HIV, or HCC, (ii) had a history of Hepatitis C treatment. Furthermore, 48 healthy controls were also recruited as a comparison group. The Ethics Committee at the Faculty of Medicine Universitas Indonesia approved the study with the number of approval letter: 85/UN2.F1/ETIK/2019. Informed consent was collected from each participant before the data collection process
Liver stiffness measurement
Liver Stiffness Measurement was assessed using Fibro Scan®, and the result was used as the reference standard. A skilled full physician performed the assessment of LSM after the measurement of serum M2BPGi on the same day. The median of ten LSM values was used as the final score. Significant fibrosis was defined as of LSM ≥8 kPA, while cirrhosis was defined as the level of LSM ≥12 kPA. Moreover, probe XL was used to assess liver fibrosis among obese patients.
erum M2BPGi measurement
M2BPGi level was assessed through a lectin-antibody sandwich immunoassay using the HISCL-5000 immune analyzer (Sysmex Corporation, Hyogo, Japan). The blood sample was taken before the measurement of LSM measurement. The final value of M2BPGi was obtained by using the following equation:
Cut Off Index (COI) = ([M2BPGi] sample – [M2BPGi]NC)/ ([M2BPGi]PC-[M2BPGi]NC) in which [M2BPGi]sample is the M2BPGi found in the serum sample, PC is a positive control, and NC is the negative control. The positive control was used as the calibration solution preliminarily standardized, which provided a cut off value of 1.0. The result of M2BPGi level is automatically calculated by the instrument [17].
Statistical analysis
Correlation between Liver Stiffness Measurement and serum M2BPGi level was analyzed using Pearson correlation. Differences between numeric variables were assessed by using independent T-test analysis for the parametric data and MannWhitney U test for the nonparametric data. Optimal serum M2BPGi cut off value was evaluated by performing ROC and AUC analysis based on the optimal sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Result
Baseline clinical characteristic of patients
The baseline of the study participants is shown in (Table 1). The enrolled patients in the case group were dominated by male participants (59%), while the control group was dominated by female participants (54%). In the Hepatitis C infected group, the median of liver stiffness measurement (LSM) was significantly higher than the healthy control group (14.3 kPA vs. 5.1 kPA, p<0.001). Most of the Hepatitis C group were in the cirrhosis stage (≥F4), accounting for 60% of the total participant in the case group. Moreover, the M2BPGi level also differed significantly between the case and control groups (4.13 COI vs. 0.59 COI, p<0.001).
Table 1: Baseline Characteristic.
Characteristic |
Cases (n = 56) |
Control (n=48) |
P value |
Sex (M/F) |
|
22/26 |
>0.05 |
LSM, kPA, median (range) |
14.35 (3.0 – 75.0) |
5.1 (3.5 – 8.7) |
<0.001 |
Fibrosis stage (F0-F1/ F2-F3 /≥F4) |
14/8/34 |
47/1/0 |
<0.001 |
ALT |
|
|
|
F0-F1 |
51.6 ± 30.2 |
- |
- |
F2-F3 |
67.5 ± 45.3 |
- |
- |
≥F4 |
63.1 ± 53.9 |
- |
- |
AST |
|
|
|
F0-F1, mean ± SD |
42.5 ± 21.9 |
- |
- |
F2-F3 mean ± SD |
52.0 ± 15.5 |
- |
- |
≥F4, mean ± SD |
65.3 ± 39.7 |
- |
- |
M2BPGi, COI, median (range) |
4.13 (0.72 – 26.00) |
0.59 (0.23 – 1.27) |
<0.001 |
Correlation between M2BPGi and liver stiffness measurement in treatment naïve chronic hepatitis C
Patients: Based on the Pearson correlation analysis, the level of serum M2BPGi was found to have a strong positive correlation with the liver stiffness measurement by Transient Elastography (r=0.708, p<0.001) (Figures 1a). Moreover, the median level of serum M2BPGi increased significantly according to the severity of liver fibrosis stages as 1.50 COI for F0-F1, 2.985 COI for significant fibrosis (F2-F3) and 8.785 COI for cirrhosis (≥F4). The analysis also showed that the level of M2BPGi between healthy control and F0-F1 Hepatitis C group differed significantly (0.585 COI vs 1.500 COI, p<0.001) (Figures 1b).
Cut-off values of serum M2BPGi to detect significant fibrosis and cirrhosis
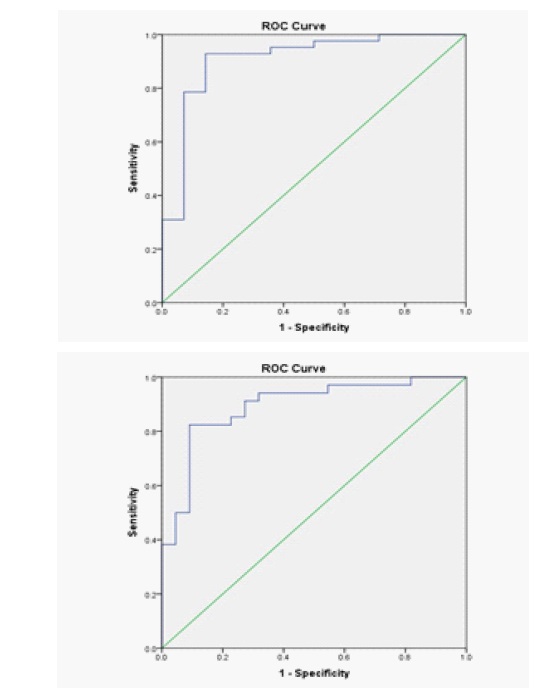
ROC analysis was carried out to evaluate the diagnostic performance of serum M2BPGi to evaluate significant liver fibrosis and cirrhosis. The AUCs were 0.908 (95%CI: 0.807 – 1.000) for evaluating significant fibrosis and
0.893 (95%CI: 0.806 – 0.980) for evaluating cirrhosis (Figure 2).
The optimal cut-off value for diagnosing significant fibrosis and cirrhosis were 1.820 COI and 3.770 COI, respectively. The sensitivity, specificity, negative predictive value, and positive predictive value for each cut-off were presented in (Table 2).
Table 2: Diagnostic Performance of Serum M2BPGi for Evaluating Significant Fibrosis and Cirrhosis.
Fibrosis Stages |
Cutoff |
AUC |
Sen |
Spe |
NPV |
PPV |
Significant Fibrosis (≥F2) |
1.820 |
0.908 (0.807- 0.000) |
0.93 |
0.86 |
0.80 |
0.95 |
Cirrhosis (≥F4) |
3.770 |
0.893 (0.806 -0.980) |
0.82 |
0.91 |
0.77 |
0.93 |
Discussion
Hepatitis C Virus infection could progress to numerous extra-hepatic manifestations and affect the achievement of SVR. However, due to its high cost, not all patients infected with the virus would undergo therapy. In this case, liver fibrosis measurement is essential in the decision-making to start treatment and determine the patients’ adequate follow-up [6]. Furthermore, the fibrosis stage has also been a primary predictive factor of complications after eradicating the virus [18]. The gold standard for assessing liver fibrosis is a liver biopsy. However, it has several limitations, such as the risk of tremendous side effects due to its invasive nature and the possibility of sampling bias also inter-observer variability [7,19]. Several non-invasive modalities have been developed to overcome this barrier, such as Transient Elastography, which is accessible, accurate, and safe for patients. It has also been widely investigated and validated; therefore, it is also recognized as the liver biopsy’s surrogate modality [20].
In this study, it was found that the level of serum Mac-2-binding Protein Glycosylation Isomer (M2BPGi) in treatment naïve Chronic Hepatitis C patients had a strong positive correlation with liver stiffness measurement by using Transient Elastography (r=0.708, p<0.001). Moreover, the level of serum M2BPGi tended to increase according to the severity of liver stiffness stages (Figures 1a-b). These findings were in line with the previous 168 study, which also found that serum M2BPGi was positively correlated with Transient Elastography (rho=0.504, 169 p<0.001) [17]. Moreover, a significant difference of serum M2BPGi level was found between the treatment naïve Chronic Hepatitis C groups and healthy control (4.13 COI vs. 0.59, p<0.001). A previous study has suggested that M2BPGi is sent by the Hepatic Stellate Cells (HSCs) as a juxtracrine-acting messenger to Kuppfer Cells during the occurrence of liver fibrosis. Hence, the alteration of serum M2BPGi between healthy control and Chronic Hepatitis C patients could demonstrate the progression of liver fibrosis which occurs among the Chronic Hepatitis C patients [11].
ROC analysis also showed an excellent diagnostic accuracy for detecting significant fibrosis (AUC: 0.908) and cirrhosis (AUC: 0.893) using the cut-off levels of 1.820 COI and 3.770 COI, respectively, with high sensitivity and specificity, especially for significant fibrosis (Table 3). Hence, this marker could be reliable for screening, distinguishing patients with significant liver fibrosis from those who do not. Although the ROC, sensitivity, and 180 specificity of serum M2BPGi for diagnosing cirrhosis was found lower than for diagnosing significant fibrosis, 181 the result was still considered to be satisfying.
Table 3: Diagnostic Performance of Serum M2BPGi for Evaluating Significant Fibrosis and Cirrhosis.
Fibrosis Stages |
Cutoff |
AUC |
Sen |
Spe |
NPV |
PPV |
Significant Fibrosis (≥F2) |
1.820 |
0.908(0.807-1.000) |
0.93 |
0.86 |
0.80 |
0.95 |
Cirrhosis (≥F4) |
3.770 |
0.893(0.806-0.980) |
0.82 |
0.91 |
0.77 |
0.93 |
Limitations
There are several limitations to this study. Firstly, there was no liver biopsy assessment, which could lead to bias, especially when the liver stiffness measurement falls into the grey area. Secondly, based on the previous studies, it was stated that the specificity of M2BPGi could be an issue due to the influence of other medical conditions, such as acute liver injury and adenocarcinoma [21,22]. However, this study also excluded participants who had been diagnosed with Hepatitis B Virus infection and hepatocellular carcinoma. Therefore, the bias could be minimized. Moreover, best to our knowledge, this is the first study about the role of M2BPGi in detecting liver fibrosis in Indonesian patients who are treatment naïve Hepatitis C Chronic.
Declarations
Ethics approval and consent to participate
Ethics Committee of The Faculty of Medicine Universitas Indonesia approved the study before the study initiation under the letter of ethical approval No: 85/UN2.F1/ETIK/2019. Each participants gave their written consent after the explanation of the study procedure and purpose.
Consent for publication: Not applicable.
Availability of data and material: The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflict of interest: Andri Sanityoso Sulaiman received research grant from the Ministry of Health of Republic Indonesia and Sysmex Indonesia Company. The funders had no role in the design pf the study, in the collection, analysis, interpretation of data, in writing the manuscript, or in the decision to publish the result.
Funding: This study was supported by the Ministry of Health of Republic Indonesia through Health Science and Technology Research Grant (No: HK.03.01/1/994/2020) and by the Sysmex Indonesia (No:PEA0006/MKT/ESI/XI/2019).
Author Contributions: ASS proposed, designed, and conducted the study. IH, CRAL, JK, COMJ, SN, and KFK performed the research. RSH collected and analyzed the data. ASS wrote the draft of the manuscript. RAG reviewed the manuscript. ASS was the research coordinator.
References
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016; 22: 7824.
- Badan Penelitian dan Pengembangan Kesehatan Kemenkes RI. Hasil Utama Riskesdas 2018 [Internet]. Ministry of Health Republic of Indonesia, 2019.
- World Health Organization, World Health Organization, Global Hepatitis Programme. Global hepatitis report, 2017.
- EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2014; 60: 392-420.
- Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020; 73: 1170-218.
- Carmona I, Cordero P, Ampuero J, Rojas A, Romero-Gómez M. Role of assessing liver fibrosis in management of chronic hepatitis C virus infection. Clin Microbiol Infect. 2016; 22: 839-45.
- Chi H, Hansen BE, Tang WY, Schouten JNL, Sprengers D, Taimr P, et al. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy: Eur J Gastroenterol Hepatol. 2017; 29: 36-41.
- Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. 2019; 13: 361-74.
- Mendes LC, Ferreira PA, Miotto N, Zanaga L, Gonçales ESL, Pedro MN, et al. Elastogram quality assessment score in vibrationcontrolled transient elastography: Diagnostic performance compared to digital morphometric analysis of liver biopsy in chronic hepatitis C. J Viral Hepat. 2018; 25: 335-43.
- Mendes L, Stucchi R, Vigani A. Diagnosis and staging of fibrosis in patients with chronic hepatitis C: comparison and critical overview of current strategies. Hepatic Med Evid Res. 2018; 10: 13-22.
- Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018; 53: 819-26.
- Ito K, Murotani K, Nakade Y, Inoue T, Nakao H, Sumida Y, et al. Serum Wisteria floribunda agglutininpositive Mac-2-binding protein levels and liver fibrosis: A meta-analysis: WFA+-M2BP and liver fibrosis. J Gastroenterol Hepatol. 2017; 32: 1922-30.
- Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity Creactive protein concentration in autoimmune hepatitis: WFA + -M2BP and hCRP in AIH. Hepatol Res. 2016; 46: 613-21.
- Yamada N, Sanada Y, Tashiro M, Hirata Y, Okada N, Ihara Y, et al. Serum Mac-2 binding protein glycosylation isomer predicts grade F4 liver fibrosis in patients with biliary atresia. J Gastroenterol. 2017; 52: 245-52.
- Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, et al. Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein Level Predicts Liver Fibrosis and Prognosis in Primary Biliary Cirrhosis: Am J Gastroenterol. 2015; 110: 857-64.
- Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 2015; 50: 776-84.
- Xu H, Kong W, Liu L, Chi X, Wang X, Wu R, et al. Accuracy of M2BPGi, compared with Fibro Scan®, in analysis of liver fibrosis in patients with hepatitis C. BMC Gastroenterol. 2017; 17: 62.
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatol Baltim Md. 2015; 62: 932-54.
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002; 97: 2614-8.
- Mak L-Y, Wong DK-H, Seto W-K, Ning Q, Cheung K-S, Fung J, et al. Correlation of serum Mac-2-binding protein glycosylation isomer (M2BPGi) and liver stiffness in chronic hepatitis B infection. Hepatol Int. 2019; 13: 148-56.
- Morio K, Imamura M, Daijo K, Teraoka Y, Honda F, Nakamura Y, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol. 2017; 52: 1252-7.
- Waragai Y, Suzuki R, Takagi T, Sugimoto M, Asama H, Watanabe K, et al. Clinical significance of serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in pancreatic ductal adenocarcinoma. Pancreatol Off J Int Assoc Pancreatol IAP Al. 2016; 16: 1044-50.