Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Impact of hepatitis C virus on cardiovascular risk among Egyptian patients on maintenance dialysis
Naglaa Elarabany1,2; Maryam A Aljohani1; Mohamed A Abdelrazek3,4*
1 Biology Department, Faculty of Science and Arts, Shaqraa University, Saudi Arabia.
2 Zoology Department, Faculty of Science, Dameitta University, Egypt.
3 Research and Development Department, Biotechnology Research Center, New Damietta, Egypt.
4 Sherbin Central Hospital, Ad Daqahliyah, Ministry of Health, Egypt.
*Corresponding Author : Abdelrazek MA
Biotechnology Research Center, 23 July St.,
Industrial Zone, New Damietta 34517, Egypt.
Email: maabdelrazek@yahoo.com
Received : Dec 06, 2021
Accepted : Jan 19, 2022
Published : Jan 26, 2022
Archived : www.jjgastro.com
Copyright : © Abdelrazek MA (2022).
Abstract
Some studies reported the association between cardiovascular disease (CVD) and hepatitis C virus (HCV) with controversial findings. However, almost no study concerned with this issue in Egyptian patients. We aimed to evaluate the association between HCV-infection and CVD development in Egyptian hemodialysis patients and to assess relation between infection and other traditional risk factors. 130 Egyptian dialysis patients (with/ without CVD) and 50 healthy sex-matched controls were included. In contrast to patients without CVD (25.3%), HCV positivity rate was significantly (P=0.0001) higher in CVD patients (67.3%). Among all studied parameters, HCV infection was significantly (r=0.415; P=0.0001) correlated with CVD development as well as body mass index, carotid thickening, dialysis duration, decreased high-density lipoproteins, elevated cholesterol, triglycerides, low- and very low-density lipoproteins. Multiple logistic regression analysis revealed that HCV infection (P=0.015) was independently associated with CVD development (OR=5.948, 95 CI (2.7-13.1)). Moreover, HCV-infected patients were significantly associated with all other CVD risk factors. In conclusion, this study highlighted the association between HCV prevalence among Egyptian patients on maintenance hemodialysis and the risk of CVD risk. Thus, further studies are needed to assess the potential role of antiviral treatments using current direct acting antiviral modalities in improving clinical outcome in Egyptian dialysis patients.
Keywords: hepatitis C virus; dialysis patients; cardiovascular risk; lipid alterations.
Abbreviations: CKD: Chronic Kidney Disease; CVD: Cardiovascular Disease; HCV: Hepatitis C Virus; ELISA: Enzyme Linked Immunosorbent Assay.
Citation: Elarabany N, A Aljohani MA, Abdelrazek MA. Impact of hepatitis C virus on cardiovascular risk among Egyptian patients on maintenance dialysis. Japanese J Gastroenterol Res. 2022; 2(2): 1054.
Introduction
Chronic kidney disease (CKD) patients have an increased risk of mortality especially patients with end-stage renal disease [1]. Remarkably, almost half of deaths are related to CVD and this high mortality give an urge to identify more modifiable risk factors to improve the survival of hemodialysis-dependent patients [2]. In addition to liver disease, chronic infection with HCV has been associated with many extrahepatic comorbidities, including renal disease, CVD, insulin resistance, diabetes mellitus, lymphoproliferative disease and cryoglobulinemia [3]. In dialysis populations, anti-HCV-positive serologic status has been reported to be significantly associated with lower survival [4] and HCV has been included in the modifiable (non-traditional) death risk factors in hemodialysis patients [5].
In contrast to the general population, hemodialysis patients have significantly higher HCV prevalence. This high prevalence is due to transmission by kidney grafts, blood transfusions (before effective HCV screening of blood donors) and nosocomial transmission in dialysis units [6]. Hemodialysis patients with HCV infection have poor vital prognosis due to cirrhosis, CVD and hepatocellular carcinoma. The association between CVD and HCV infection is still an issue of controversy. Some studies have reported higher CVD risk among HCV-infected patients [7] whereas others have not demonstrated such association [8]. HCV role in CVD development was thought to be related to interference with lipid and glucose metabolism that may cause metabolic syndrome development and subsequent risk of developing CVD, type 2 diabetes and insulin resistance. Beyond this, HCV was also thought to increase CVD risk via mechanisms that facilitate endothelial dysfunction and/or chronic inflammation [9, 10]. Almost, there are no studies that assessed the association between HCV infection coexistence and the CVD development in CKD Egyptian patients on maintenance hemodialysis. In this study, we aimed to evaluate the association between HCV infection and CVD development in Egyptian hemodialysis patients and to assess the relation between infection and other traditional CVD risk factors.
Materials and methods
Patients
Participants were 130 Egyptian CKD patients who were receiving thrice weekly chronic hemodialysis. Mean age was 50.0 ± 10.5 years and there were 69 males and 61 females. Eligible patients were women and men, >18 years old, who were undergoing hemodialysis. HCV patients with liver cirrhosis or patients with any other chronic diseases or cancers were not eligible. In addition, 50 healthy sex-matched individuals were enrolled as controls. Retrospectively, patient’s data were recorded from medical files of each patient. This study was designed according to Helsinki Declaration ethical guidelines.
aboratory and biomarker measurements
Blood samples were collected and centrifuged at 1000 ×g for 15 minutes and stored at −20°C until analysis. Using fresh serum, kidney and liver function tests and lipid profile were measured on fully automated Biochemistry analyzer (Response 920, Diasys Diagnostic Systems, GmbH, Germany) and minerals were determined using blood gas/electrolyte analyzer (GEM Premier 3000, Werfen, Belgium). KEDTA treated blood was used for complete blood count on automated hematology analyzer (Phoenix NCC-3300, NeoMedica Bioscience Technology, Serbia). HCV antibodies were detected by using commercial ELISA kits (abia HCV-Ab, AB Diagnostic Systems, GmbH, Germany).
Statistical analysis
Data analysis was performed using GraphPad Prism (v. 6) and SPSS (v. 20) software. Normally and non-normally distributed data are shown as mean ± standard deviation (SD) and median (interquartile range), respectively. ANOVA and student t test were used to compare values between groups with normal distribution. In case of skewed variable distribution, KruskalWallis test was used. Proportions in groups were compared using Pearson χ2 test. P<0.05 was considered statistically significant. Multiple logistic regression was used to estimate HCV odd ratio (OR) and 95% confidence intervals (95% CIs) for CVD development. Spearman’s rank correlation analysis was used to assess the association between any two variables.
Factors associated with CVD
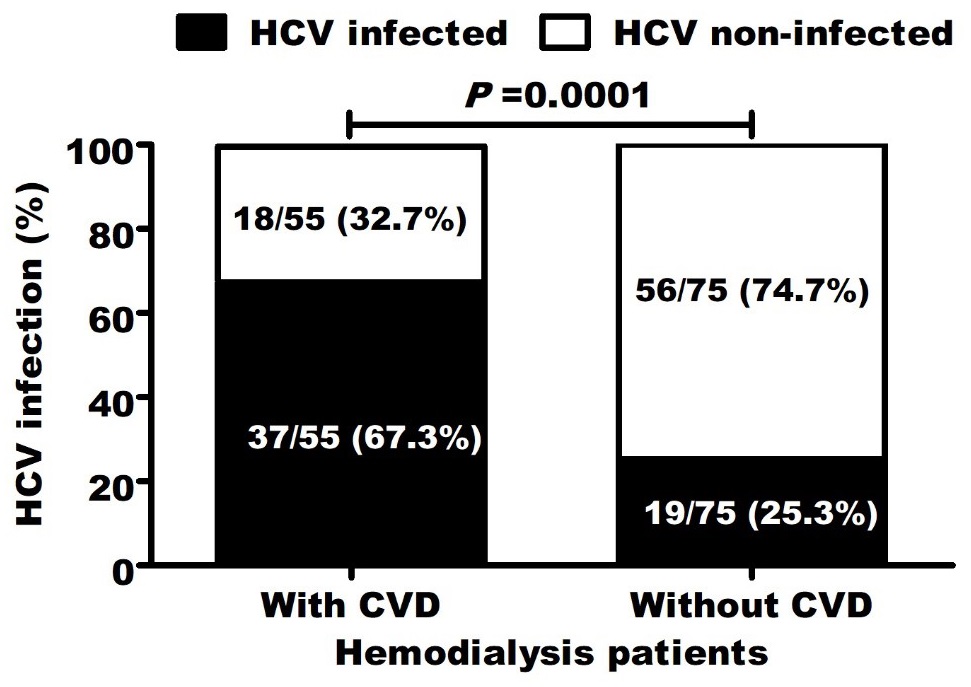
Among all studied parameters, CVD development was significantly (P<0.05) correlated with increased body mass index, carotid thickening, long dialysis duration, decreased high-density lipoproteins, elevated cholesterol, triglycerides, low- and very low-density lipoproteins and HCV infection (r=0.415; P=0.0001) (Table 2).
Results
Characteristics of patients: Demographic and clinical characteristics of patients and controls were summarized in (Table 1). Hemodialysis patients with CVD were older (P=0.0351) than patients without CVD and healthy controls. Besides creatinine and urea blood levels, hemodialysis patients were associated with elevated levels of uric acid, sodium and potassium and decreased level of albumin. They also had overweight and microcytic anemia. Patients with CVD were associated with long dialysis duration, carotid intima thickening and dyslipidemia, including decreased levels of high-density lipoprotein and elevated levels of cholesterol, triglycerides, low density lipoprotein and very low-density lipoprotein.
HCV infection independently associated with CVD
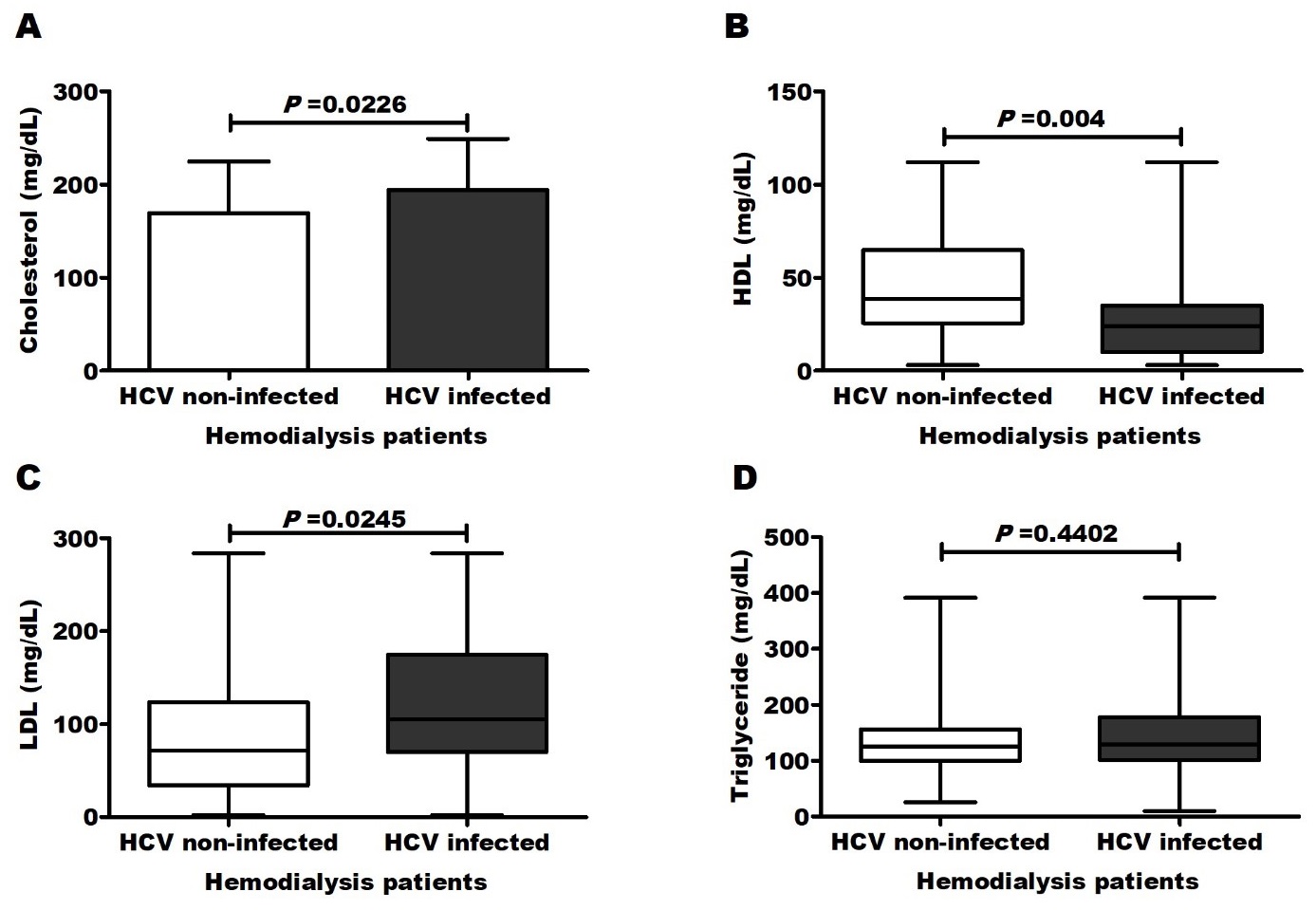
Screening for HCV infection showed that 37/55 (67.3%) of patients with CVD and 19/75 (25.3%) of patients without CVD were anti-HCV-positive. The positivity rate was significantly (P=0.0001) higher in CVD patients (Figure 1). In multiple logistic regression analysis, only HCV infection (P=0.015) as well as carotid thickening (P=0.002), long dialysis duration (P=0.006) and decreased high-density lipoproteins (P=0.001) still significantly related to CVD development. The odd ratio (OR=5.948, 95 CI (2.7-13.1), P<0.0001) for CVD increased in patients with HCV infection compared to uninfected patients. Moreover, HCV-infected patients were significantly associated with all other CVD risk factors (Figure 2 and Table 4).
Table 1: Patient’s characteristics.
Parameter |
Healthy |
Hemodialysis patients |
P value |
|
Without CVD |
With CVD |
|||
Number |
50 |
75 |
55 |
ـــــ |
Age (Years) |
35.6±10.5 |
39.6±11.8 |
59.9±13.7 |
0.0351 |
Gender (male/female) |
30/20 |
40/35 |
29/26 |
0.409 |
BMI |
21.5±3.7 |
22.1±3.7 |
28.7±4.9 |
<0.0001 |
Pre-dialysis creatinine (mg/dL) |
0.7±0.1 |
9.53±2.8 |
9.65±3.5 |
<0.0001 |
Post-dialysis creatinine (mg/dL) |
ـــــ |
2.9±1.0 |
3.4±1.2 |
0.364 |
Pre-dialysis urea (mg/dL) |
20.0±2.64 |
140.0±39.0 |
130.4±38.0 |
<0.0001 |
Post-dialysis urea (mg/dL) |
ـــــ |
44.2±10.5 |
43.2±11.0 |
0.825 |
Uric acid (mg/dL) |
3.5±1.4 |
5.4±1.9 |
6.6±1.9 |
<0.0001 |
ALT (U/L) |
20.1±1.4 |
33.3±9.5 |
35.3±10.0 |
0.045 |
AST (U/L) |
19.2±3.3 |
29.2±9.9 |
35.0±8.9 |
0.047 |
Albumin (g/dL) |
4.3±0.53 |
3.6±0.50 |
3.5±0.25 |
0.002 |
Bilirubin (mg/dL) |
0.7±0.1 |
0.8±0.1 |
1.0±0.15 |
0.048 |
Sodium (mmol/L) |
131.7±1.58 |
137.9±3.65 |
138.3±3.63 |
0.0851 |
Potassium (mmol/L) |
3.95±0.56 |
4.76±1.18 |
4.76±1.40 |
0.0914 |
Dialysis duration (Years) |
ـــــ |
2.8±1.5 |
6.2±2.5 |
<0.0001 |
0.73±0.02 |
1.23±0.02 |
1.51±0.08 |
<0.0001 |
|
Cholesterol (mg/dL) |
150.0±35.6 |
164.0±47.6 |
196.1±60.0 |
<0.0001 |
Triglycerides (mg/dL) |
99 (53-125) |
129 (99-160) |
140 (111-200) |
0.0589 |
HDL (mg/dL) |
57 (47-69) |
50 (35-76) |
15 (9-30) |
<0.0001 |
LDL (mg/dL) |
44 (20-52) |
65 (31-110) |
110 (98-185) |
<0.0001 |
vLDL (mg/dL) |
22 (12-24) |
30 (20-35) |
40 (30-50) |
0.0025 |
Hemoglobin (g/dL) |
12.0±2.1 |
10.0±1.8 |
9.7±1.7 |
0.042 |
White blood cells (109/L) |
5.5±1.8 |
6.4±2.2 |
5.6±2.0 |
0.156 |
Red blood cells (1012/L) |
4.0±0.92 |
3.5±0.80 |
3.6±0.73 |
0.025 |
Platelets (109/L) |
200.79±55.21 |
152.88±40.51 |
145.82±50.51 |
0.034 |
Abbreviations: BMI: Body Mass Index; ALT, AST: Alanine And Aspartate Aminotransferases; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very High-Density Lipoprotein. Normally distributed variables were expressed as mean±SD. Non-normally distributed variables were expressed as median (interquartile range). Significant difference was determined using X2 test, ANOVA and Kruskal-Wallis tests as appropriate. P<0.05 was considered significant.
Table 2: Correlation between CVD development and other clinical risk factors.
Factor correlated |
Correlation coefficient (r) |
P value |
Body mass index |
0.550 |
0.0001 |
Carotid thickness (mm) |
0.425 |
0.0001 |
Dialysis duration (Years) |
0.645 |
0.0001 |
Cholesterol (mg/dL) |
0.262 |
0.005 |
Triglycerides (mg/dL) |
0.203 |
0.035 |
HDL (mg/dL) |
-0.821 |
0.0001 |
LDL (mg/dL) |
0.522 |
0.0001 |
vLDL (mg/dL) |
0.350 |
0.0001 |
HCV-infection |
0.415 |
0.0001 |
Abbreviations: CVD: Cardiovascular Diseases; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very High-Density Lipoprotein.
Table 3: Parameters independently associated with CVD development.
Factor |
Beta |
Standard error |
95% CI for Beta |
P Value |
Body mass index |
0.012 |
0.008 |
-0.004 - 0.028 |
0.153 |
Carotid thickness (mm) |
0.017 |
0.009 |
0.008 - 0.032 |
0.002 |
Dialysis duration (Years) |
0.037 |
0.013 |
0.011 - 0.064 |
0.006 |
Cholesterol (mg/dL) |
0.000 |
0.001 |
-0.001 - 0.002 |
0.664 |
Triglycerides (mg/dL) |
-0.002 |
0.001 |
-0.004 - 0.000 |
0.057 |
HDL (mg/dL) |
-0.009 |
0.001 |
-0.012 - -0.006 |
0.0001 |
LDL (mg/dL) |
0.000 |
0.001 |
-0.001 - 0.002 |
0.576 |
vLDL (mg/dL) |
0.001 |
0.005 |
0.004 - 0.022 |
0.056 |
HCV-infection |
0.104 |
0.062 |
0.031 – 0.278 |
0.015 |
Abbreviations: CVD: Cardiovascular Diseases; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very High-Density Lipoprotein.
Table 4: Correlation between HCV-infection and CVD related clinical risk factors.
Factor correlated |
Correlation coefficient (r) |
P value |
CVD development |
0.415 |
0.0001 |
Body mass index |
0.596 |
0.0001 |
Carotid thickness (mm) |
0.326 |
0.0001 |
Dialysis duration (Years) |
0.387 |
0.0001 |
Cholesterol (mg/dL) |
0.254 |
0.010 |
Triglycerides (mg/dL) |
0.112 |
0.249 |
HDL (mg/dL) |
-0.375 |
0.0001 |
LDL (mg/dL) |
0.245 |
0.015 |
vLDL (mg/dL) |
0.172 |
0.073 |
Abbreviations: CVD: Cardiovascular Diseases; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein; VLDL: Very High-Density Lipoprotein.
Discussion
Despite HCV prevalence reduction, hemodialysis patients still comprise a high risk group [11]. In hemodialysis population, true HCV impact on survival is difficult to assess and these patients may die from other causes, mainly CVD, before developing liver cirrhosis or hepatoceluular carcinoma [11]. It stills unclear whether elevated mortality risk in HCV infected patients on hemodialysis is related only to a higher rate of liver-related diseases or to another comorbidities in HCV-infected patients on dialysis [12]. Here, we aimed to evaluate the prevelance of CVD in Egyptian hemodialysis patients with and without HCVinfection. Also, we aimed to assess the relation between HCV infection and CVD traditional risk factors including interference with lipids.
Here and among all studied parameters, CVD development was significantly correlated with HCV infection (r=0.415; P=0.0001) as well as other CVD traditional risk factors in dialysis patients including increased body mass index [13], carotid thickening [14], long dialysis duration [15] and lipid alterations including decreased high-density lipoproteins and elevated cholesterol, triglycerides, low- and very low-density lipoproteins [16]. Compared to uninfected patients (25.3%), high percentage (67.3%) of HCV-infected patients was with CVD.
HCV infection results in systemic and hepatic inflammation and it has been suggested that indirect and direct mechanisms can be involved CVD development via inducing pro-atherogenic metabolic factors and increased levels of pro-atherogenic cytokines and chemokines [17]. Carefully, the relationships between immune response to HCV infection and CVD have been examined [18]. In contrast to uninfected patients, patients with HCV infection were associated with higher TNF-α level [18], more pronounced intima-media thickness [19], higher levels of proinflammatory cytokines and ratio of proinflammatory/antiinflammatory cytokines [20] and higher risk of cardiac failure and death [18]. Also, cryoglobulinemia occurs in many HCV infected patients and the presence of cryoglobulinemia is associated with cardiovascular and vasculitis events [21]. Besides HCV role in chronic inflammation development involving the arteries, sequences of HCV-RNA have been detected within carotid plaques, suggesting consequent local pro-atherogenic action [17].
In this study, multiple logistic regressions, that adjusted other demographic and clinical characteristics, revealed that HCV infection (P=0.015) was independently related to CVD development [OR=5.948, 95 CI (2.7-13.1)]. Interestingly, HCV-infected patients were significantly associated with other CVD risk factors including increased body mass index, carotid thickening, long dialysis duration and dyslipidaemia.
After adjustment for several covariates including age, diabetes mellitus, dialysis vintage, gender, age and others, the persistence of an increased HCV-associated CVD risk was observed [12]. In HCV infected hemodialysis patients (n=2,778), Kalantar-Zadeh et al. were the first researchers to establish greater CVD risk. After adjustment for case-mix and measures of malnutrition-inflammation complex syndrome, they reported that HCV positivity among patients <65 years was associated with 40-80% higher hazard ratio of cardiovascular death [22]. Other study on a large cohort of HCV-infected patients treated with haemo- and peritoneal dialysis found that coronary artery disease was an independent mortality risk factor [23, 24]. In patients undergoing regular dialysis, coronary flow reserve was significantly lower in HCV-infected than uninfected patients [25]. By multiple logistic regression analysis, Oyake et al. found that mean blood pressure and HCV infection were significantly and independently associated with high aortic pulse wave velocity [26]. In accordance with our findings, HCV infection was reported to be associated with traditional CVD risk factors including body mass index [27], carotid intima-media thickening [28] and dyslipidaemia [29].
Conclusions
In conclusion, this study highlighted the association between the prevalence of HCV and CVD risk and its related risk factors among Egyptian patients on maintenance hemodialysis. These results may have implications not only in HCV management in these patients but it shed the light on the potential role of antiHCV treatment using current direct acting antiviral modalities in improving clinical outcome in Egyptian dialysis patients.
References
- Wang F, Yang C, Long J, Zhao X, Tang W, Zhang D, et al. Executive summary for the 2015 Annual Data Report of the China Kidney Disease Network (CK-NET). Kidney Int. 2019; 5: 501-505.
- Lin Z, Qin X, Yang Y, Huang Y, Wang J, Kong Y, et al. Higher dietary fiber intake is associated with lower cardiovascular disease mortality risk among maintenance hemodialysis patients: a multicenter prospective cohort study. Br J Nutr. 2021; 1-25.
- Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, Bagchi S. Risk of Cardiovascular Disease Due to Chronic Hepatitis C Infection: A Review. Journal of clinical and translational hepatology. 2017; 5: 343-362.
- Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012; 19: 601-607.
- Fabrizi F, Donato FM, Messa P. Hepatitis C and Its Metabolic Complications in Kidney Disease. Ann Hepatol . 2017; 16: 851- 861.
- Miyazaki R, Miyagi K. Effect and Safety of Daclatasvir-Asunaprevir Combination Therapy for Chronic Hepatitis C Virus Genotype 1b -Infected Patients on Hemodialysis. Ther Apher Dial. 2016; 20: 462-467.
- Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012; 32: 421-430.
- Pothineni NV, Rochlani Y, Vallurupalli S, Kovelamudi S, Ahmed Z, Hakeem A,Mehta JL. Comparison of Angiographic Burden of Coronary Artery Disease in Patients With Versus Without Hepatitis C Infection. Am J Cardiol. 2015; 116: 1041-1044.
- Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol. 2014; 61: S69-78.
- Badawi A, Di Giuseppe G, Arora P. Cardiovascular disease risk in patients with hepatitis C infection: Results from two general population health surveys in Canada and the United States (2007-2017). PLoS One. 2018; 13: e0208839.
- Marinaki S, Boletis JN, Sakellariou S, Delladetsima IK. Hepatitis C in hemodialysis patients. World J Hepatol. 2015; 7: 548-558.
- Fabrizi F, Messa P, Martin P. Recent advances on hepatitis C virus in dialysis population. Kidney Blood Press Res. 2014; 39: 260- 271.
- Nishizawa Y, Shoji T, Ishimura E. Body composition and cardiovascular risk in hemodialysis patients. J Ren Nutr. 2006; 16: 241- 244.
- Abbasi MR, Abbaszadeh SH, Rokni-Yazdi H, Lessan-Pezeshki M, Khatami MR, Mahdavi-Mazdeh M, et al. Carotid intima-media thickness as a marker of atherosclerosis in hemodialysis patients. Ind J Nephrol. 2006; 26: 97-101.
- Yuenyongchaiwat K, Vasinsarunkul P, Phongsukree P, Chaturattanachaiyaporn K, Tritanon O. Duration of hemodialysis associated with cardio-respiratory dysfunction and breathlessness: a multicenter study. Peer J. 2020; 8: e10333.
- Qunibi WY. Dyslipidemia in Dialysis Patients. Semin Dial. 2015; 2: 345-353.
- Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, et al. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013; 5: 528-540.
- Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fai. 2018; 15: 451-456.
- Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, et al. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010; 59: 1135-1140.
- Oliveira CP, Kappel CR, Siqueira ER, Lima VM, Stefano JT, Michalczuk MT, et al. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013; 164: 221-226.
- Ragab G, Hussein MA. Vasculitic syndromes in hepatitis C virus: A review. J Adv Res. 2017; 8: 99-111.
- Kalantar-Zadeh K, McAllister CJ, Miller LG. Clinical characteristics and mortality in hepatitis C-positive haemodialysis patients: a population based study. Nephrol Dial Transplant. 2005; 20: 1662-1669.
- Bose B, McDonald SP, Hawley CM, Brown FG, Badve SV, Wiggins KJ, et al. Effect of dialysis modality on survival of hepatitis C-infected ESRF patients. Clin J Am Soc Nephrol. 2011; 6: 2657-2661.
- Butt AA, Khan UA, Skanderson M. Comorbidities and their impact on mortality in HCV and HCV-HIV-coinfected persons on dialysis. J Clin Gastroenterol. 2008; 42: 1054-1059.
- Yelken B, Gorgulu N, Caliskan Y, Elitok A, Cimen AO, Yazici H, et al. Association between chronic hepatitis C infection and coronary flow reserve in dialysis patients with failed renal allografts. Transplant Proc. 2009; 41: 1519-1523.
- Oyake N, Shimada T, Murakami Y, Ishibashi Y, Satoh H, Suzuki K, et al. Hepatitis C virus infection as a risk factor for increased aortic stiffness and cardiovascular events in dialysis patients. J Nephrol. 2008; 21: 345-353.
- Jabeen S, Rasheed A, Jabeen N, Naz SA, Raza A. Prevalence and association of HBV and HCV infection with cardiovascular disease risk factors in a peri-urban population. J Pak Med Assoc. 2020; 70: 58-63.
- Ishizaka N, Ishizaka Y, Takahashi E, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002; 359: 133-135.
- Murthy GD, Vu K, Venugopal S. Prevalence and treatment of hyperlipidemia in patients with chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 2009; 21: 902-907.