Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Secondary prevention of gastric cancer: An alternative strategy
Calvo B Alfonso1,2*; Díaz F Alfonso1; Báez V Sergio1,2; Icaza N Gloria3; Nuñez-Franz Loreto4; Norero M Enrique1,5; Pruyas A Martha6; Nilsen N Eva6; Aguayo B Gloria6 ; Le Cerf Patricio6
1 Department of Surgery, Dr. Sótero del Río Hospital, SSMSO, Santiago de Chile, Chile.
2 Digestive Endoscopy Unit, Dr. Sótero del Río Hospital, SSMSO, Santiago de Chile, Chile.
3 Institute of Mathematics and Physics, Universidad de Talca (University of Talca), Talca, Chile.
4 Department of Public Health, School of Health Sciences, Universidad de Talca, Talca, Chile.
5 Department of Digestive Surgery. Pontifical Catholic University of Chile). Santiago, Chile.
6 Department of Pathological Anatomy, Dr. Sótero del Río Hospital. SSMSO. Santiago de Chile. Chile.
*Corresponding Author : Alfonso Calvo B
Department of Digestive Endoscopy, Dr. Sótero
del Río Hospital, Avenida Concha y Toro 3459,
Puente Alto, RM, Chile.
Email: acalvobelmar@yahoo.es
Received : Dec 05, 2021
Accepted : Jan 17, 2022
Published : Jan 24, 2022
Archived : www.jjgastro.com
Copyright : © Alfonso CB (2022).
Abstract
Background: Early detection and treatment provide the only curative possibility for gastric cancer. A cost-effective strategy is imperative in developing countries with high mortality rates. The objective of this study is to analyse the results of an organized endoscopic screening in a symptomatic population 40 years and older.
Methods: This was a prospective observational study of patients with gastrointestinal symptomology referred by primary care who had direct access to an endoscopy unit at the secondary referral centre. Physicians were trained, and an endoscopy request form and a flowchart for referral and patient management were designed. Endoscopic performance, early detection rates, resectability and survival, overall and disaggregated by sex, were evaluated.
Findings: From July 1, 1996, to June 30, 2013, 25.304 endoscopies were performed, of which 14.521 were in individuals in risk groups (70% women). A total of 339 gastric cancers were detected, for a detection rate of 2.3%. The rate of early gastric cancer (EGC) among all cancers was 34% (f: 46.6%, m: 27.6%). The resectability for both sexes was 68.1% (f: 78.8%, m: 62.4%). The EGC rate in resected patients was 45% (f: 54.8%, m: 38.4%). The five-year survival rate for all cancers was 38.1% (f: 51.7%, m: 30.8%), and that for patients who underwent resection was 57.6% (f: 69.0%, m: 50.0%). The 10-year survival for all cancers was 27.5% (f: 40.5%, m: 20.5%), and that for patients who underwent resection was 43.1% (f: 55.0%, m: 35.0%).
Interpretation: Organized screening in a symptomatic population aged 40 years and older by endoscopy has high performance, increases the rate of EGC and impacts survival. Women achieved results similar to those reported by countries with mass survey. For men to achieve these results, it is necessary to educate the population at risk, especially the most vulnerable.
Keywords: gastric cancer; endoscopic screening; resectability; early detection; survival.
Citation: Alfonso CB, Alfonso DF, Sergio BV, Gloria IN, Loreto NF, et al. Secondary prevention of gastric cancer. An alternative strategy. Japanese J Gastroenterol Res. 2021; 1(1): 1053.
Introduction
Gastric cancer (GC) continues to be a public health problem worldwide. In the last century, there was a significant decrease in mortality rates, especially in developed countries, hypothesized to be due to changes in dietary habits and food preservation [1]. This natural phenomenon also occurred in Chile, with the crude mortality rate decreasing until the 1980s, and since has stabilized at approximately 20 per 100.000 inhabitants, with slight decreased in recent years. GC is the leading cause of cancer death in Chile, with more than 3.000 deaths annually [2]. Despite the decline in the mortality rate worldwide, it is the third leading cause of cancer death, with significant geographical variations, with the highest rates in East Asia, Central and Eastern Europe and Latin America, with Chile ranking first place in Latin America and seventh worldwide [3]. These geographical variations in mortality are not only between countries but also within the same country and in Chile, the highest rates correspond to the south-central zone, and the lowest rates correspond to the extreme regions [4]. It is twice as frequent in men than in women, and although it can occur at any age, its frequency in people under 40 is low, and from that age, it begins to increase, reaching its highest incidence after 60 years of age.
In the West, the prognosis is bleak, mainly due to late diagnosis, with 5-year survival slightly higher than 10%, especially those based on population records [5, 6]. In 1962, an important milestone in the history of GC was marked when the Japanese Society of Gastroenterological Endoscopy defined and classified early gastric cancer (EGC) [7]. Detection in these stages means cure for the majority of patients with a 5-year survival of 90% or more [8]. The symptoms in these initial stages have been extensively studied, and it is estimated that approximately 50% evolve with discomfort similar to benign pathology [9]. Additionally, these lesions have cycles of ulceration and healing, and the symptoms can be alleviated with the use of antiulcer medication delaying the diagnosis [10,11]. Currently, only Japan and Korea have national programmes for GC screening with significant early detection rates, but these programmes are impractical in developing countries with high mortality rates due to a lack of human and technological resources. In these countries, it is imperative to seek cost-effective secondary prevention strategies since early diagnosis not only improves survival but also considerably reduces health costs [12]. The objective of this study is to analyse the results of an organized endoscopic screening in a symptomatic population aged 40 years and older who had direct access from primary care to an endoscopy unit in a secondary care center, evaluating endoscopic performance, early detection rates, resectability and survival, both overall and disaggregated by sex.
Material and methods
Design: GC research programme from July 1, 1996, to June 30, 2013.
Context: The commune of La Florida, located in the capital of Chile, is the third most populous commune in the country, with 365.674 inhabitants according to the 2002 census. The population of 40 years and older, insured within the public health sector, was estimated at 89.842 people. The crude GC mortality rate of the commune in the 1992-1996 period was 11.1 per 100 thousand inhabitants.
In 1996, an endoscopy unit was created in a secondary care centre (SCC) dedicated to meeting the demand of primary care centres of the commune, which belong to the public health network of the South-East Metropolitan Service (SSMSO). The project was presented to the authorities of the SSMSO and SCC who gave their approval. Primary care physicians were trained, and a flowchart was established for the referral of patients and actions to be taken according to the endoscopy results [Appendix 1] [Flowchart]. Patients were referred from all primary care clinics in the commune through a request specially designed for this purpose [Appendix 2] [Endoscopy referral form]. This request allowed the standardization of the gastroenterological questioning and to adequately select the patients in the primary level and in the endoscopy unit, prioritizing the performance of the examination according to the symptoms referred to in said sheet.
Participants: A risk group was defined as people aged 40 years and older who presented epigastralgia lasting more than 15 days, accompanied or not by ominous symptoms of GC and with or without other gastrointestinal symptoms.
Characteristics of the programme: The endoscopy unit had a procedure room and a recovery room. In the first five years (1996-2000) Olympus GIF E and P3 fibre optic equipment were used; in 2002, they were replaced by an Olympus Exera 160 videoendoscope, and in 2011, an Olympus Exera 180 videoendoscope was incorporated. All patients received local oropharyngeal anaesthesia, intravenous antispasmodics and sedation with midazolam, except for some contraindications. The biopsies were analysed in the Department of Pathology of Dr. Sótero del Río Hospital, holding regular meetings with the pathologists, especially for early lesions and those in which there was discordance between endoscopy and histology. Patients diagnosed with GC were referred to the Department of Surgery of that hospital. Seven patients were treated in other hospitals. In all resected patients, including the latter, the therapeutic procedure performed and the result of the histological study were known. All patients undergoing endoscopies were prospectively incorporated into a database and another database for patients with GC. Complete follow-up of the GC series was performed through the hospital cancer registry, clinical records, telephone contact and Civil Registry, ending 08/31/2018, with a minimum follow-up of 5 years. At the end 86 patients were alive (64 EGC and 22 AGC). In 1996, in Chile, ethical regulation was verbal between the professional and the patient. In 2012, the Law on the duties and rights of patients was enacted, and informed consent began to be used in clinical procedures.
Statistical analysis. To evaluate endoscopic performance, early detection rates, and resectability, differences between proportions were compared using Fisher’s exact test. Survival was calculated with the Kaplan-Meier method. The survival curves were compared with the log-rank test. A significance level of 5% is used. The analyses were performed with SAS version 9.3 and R 3.6.1.
Programme flowchart
Results
In the study period, 24.304 endoscopies were performed, of which 14.521 were in patients of the so-called risk group, 70% women. In these, 339 GCs were diagnosed (221 men and 118 women) (Table 1). The endoscopic performance was 2.3%, one cancer being diagnosed for every 43 patients examined, which was higher in men than in women (5.1% versus 1.2%) (p < 0.001). When considering only EGCs, the performance was 0.8% (one EGC for every 125 patients), which was also higher in men than in women. Of the total number of diagnosed GCs, 116 were classified as EGCs, including 12 unresected EGCs, with an EGC rate of 34.2%, which was higher in women than in men, 46.6% vs 27.6%, respectively (p <0.0007) (Table 2). In 231 patients, resection was performed with a resectability rate of 68.1%, which was higher in women (78.8%) than in men (62.4%) (p = 0.002). In relation to lesion depth, 104 were histologically confirmed as EGCs (53 men and 51 women); of these, 82 had only mucosal involvement (T1a), representing 78.9% of the EGCs. The EGC rate in resected patients was 45.0%, which was higher in women (54.8%) than in men (38.4%) (p <0.016). In advanced gastric cancer (AGC) at the same depth level, there were no significant differences; however, when grouping them (T2 to 4b), the percentage of men was 55.8% and women 40.9% (p = 0.032), a difference that confirms a delayed diagnosis in men (Table 3).
Thirty-four patients, both advanced and early, were not treated, 22 with comorbidity considered high risk for treatment and 12 refused (6 men and 3 women). In 9 patients macroscopically AGC and predominantly men, the causes of unresectability could not be established. In AGC, 65 were disseminated at the time of the diagnosis, 51 men and 14 women (Table 4). Twelve disseminated patients (8 men and 4 women) underwent palliative surgery, so the total disseminated at the time of diagnosis was 26.7% men (59/221) and 15.3% women (18/118), with significant difference (p = 0.02).
When dividing the 17 years of the programme into three periods, coinciding with the incorporation of better endoscopy equipment, a progression of the EGC rate was observed in women, both in total cancers, from 36.0% in the first period to 54.3% in the third, as in the resected, from 36.4% to 64.9%; in contrast, this increase did not occur in men (Figure 1).
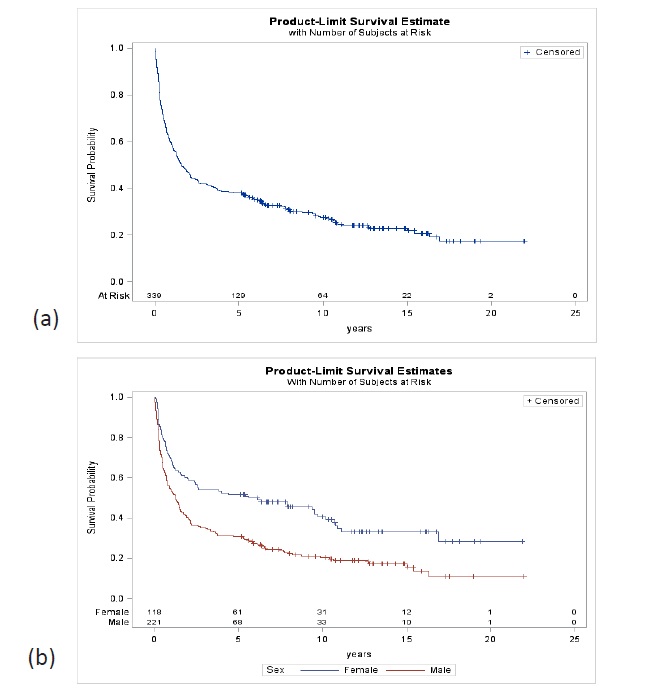
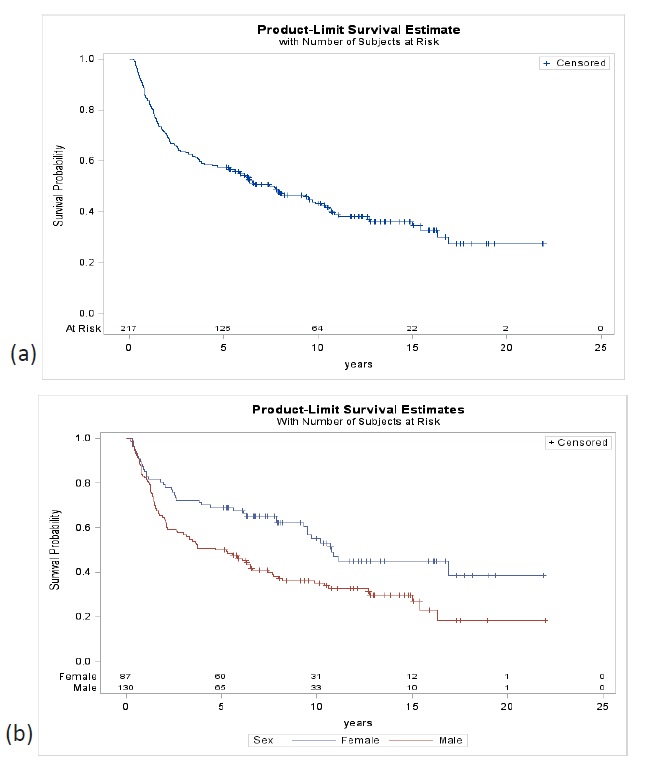
The 5-year survival of all GC patients, with a median followup of 1.6 years, was 38.1% (95% CI 33.2-43.6), 30.8% in men (95% CI 25.2-37.5) and 51.7% in women (95% CI 43.3-61.5). At 10 years, the probability of survival was 27.5% for both sexes (95% CI 22.9-32.9), 20.5% in men (95% CI 15.6-26.8) and 40.5% in women (95% CI 32.1-51.2) (Figure 2). In resected patients, with a median follow-up of 7.7 years, the 5-year survival for both sexes were 57.6% (95% CI 51.4-64.6), 50.0% in men (95% CI 42.1- 59.4) and 69.0% in women (95% CI 59.9-79.4). At 10 years, survival was 43.1% (95% CI 36.7- 50.6) for both sexes, 35% in men (95% CI 27.4-44.6) and 55% in women (95% CI 44.8-67.6) (Figure 3).
An additional benefit of this project was the finding of other malignant tumours (125). Of the total malignant neoplasms of gastric origin, 6.4% were lymphomas, and 3.5% were neuroendocrine tumours. In other locations, oesophageal cancer was the most frequent. In 15 patients with gastric metastases and other tumours, the endoscopic findings complemented with other exams allowed the primary site to be identified (Table 5).
Table 1: Population studied and total gastric cancer detected.
Men |
Women |
Total |
|
Number of endoscopies |
4324 |
10197 |
14521 |
Advanced Gastric Cancer |
160 |
63 |
223 |
Early Gastric Cancer |
61 |
55 |
116 |
Total Gastric Cancer |
221 |
118 |
339 |
Table 2: Performance and EGC rate for all gastric cancers.
|
Men % |
Women % |
Both Genders % |
p value |
Endoscopic Performance |
|
|
|
|
Total Cancer Detected |
5.1 |
1.2 |
2.3 |
<0.001 |
Early Gastric Cancer Detected |
1.4 |
0.5 |
0.8 |
< 0·001 |
|
|
|
|
|
EGC Rate |
27.6 |
46.6 |
34.2 |
<0.0007 |
Table 3: Resectability, depth of lesion and EGC rates in resected patients.
|
Men |
Women |
Total |
|
|||
n |
% |
n |
% |
n |
% |
p value |
|
Resectability |
138 |
62.4 |
93 |
78.8 |
231 |
68.1 |
0.002 |
Depth of lesion (T): |
|
|
|
|
|
|
|
T1a mucosa (m) |
44 |
31.9 |
38 |
40.8 |
82 |
35.5 |
0.2069 |
T1b submucosa (sm) |
9 |
6.5 |
13 |
14.0 |
22 |
9.5 |
0.0692 |
T2 muscularis propria (mp) |
9 |
6.5 |
5 |
5.4 |
14 |
6.1 |
0.7861 |
T3 subserosa (ss) |
11 |
8.0 |
6 |
6.5 |
17 |
7.4 |
0.7994 |
T4a serosa (se) |
54 |
39.1 |
27 |
29.0 |
81 |
35.1 |
0.1240 |
T4b serosa + neighbouring tissue |
3 |
2.2 |
0 |
0 |
3 |
1.3 |
0.2753 |
M1 |
8 |
5.8 |
4 |
4.3 |
12 |
5.2 |
0.7664 |
EGC |
53 |
38.4 |
51 |
54.8 |
104 |
45.0 |
0.0160 |
T according to Japanese classification of gastric carcinoma: 3rd English edition |
|||||||
Table 4: Causes of non-resectability.
|
Advanced |
Early |
Total |
||||
Female |
Male |
Subtotal |
Female |
Male |
Subtotal |
||
HRPC (*) |
5 |
10 |
15 |
2 |
5 |
7 |
22 |
Refused |
1 |
6 |
7 |
2 |
3 |
5 |
12 |
Disseminated |
14 |
51 |
65 |
NA |
NA |
NA |
65 |
Unknown |
1 |
8 |
9 |
NA |
NA |
NA |
9 |
Total |
21 |
75 |
96 |
4 |
8 |
12 |
108 |
(*) HRPC: High risk patient due to comorbidities |
|||||||
Table 5: Other malignant neoplasms.
Location |
n |
Stomach |
|
Lymphomas |
24 |
Neuroendocrine |
13 |
Oesophagus |
|
Squamous cell |
53 |
Adenocarcinoma |
7 |
High Grade Dysplasia (Ca in situ) |
3 |
Duodenum |
|
Neuroendocrine |
7 |
Adenocarcinoma |
1 |
Ampulla of Vater |
2 |
Gastric metastasis |
3 |
Other |
12 |
Total |
122 |
Discussion
This is a prospective observational study whose objective was to evaluate important variables in an organized GC screening programme through endoscopic of the symptomatic population in a specific geographic area, whose patients referred from primary care had direct access to an endoscopy unit in a SCC.
Mass surveys in Japan and Korea have defined all individuals aged 40 and over as a target population, which represents a high percentage of the total population. By focusing the study on symptomatic patients and specifically epigastric pain as the most important symptom, this would reduce the size of the risk group since it is estimated that the prevalence of dyspepsia in the population is approximately 25% [13].
In Japan, these studies began in 1960 using photofluorography as an initial selection method of study; however, over time, the use of this method has declined, and in 2012, participation was only 5%. In the 2000s, endoscopy began to be applied as an alternative to radiology in several centres, and in 2016, the government incorporated it as an additional screening method for GC [14]. In a study conducted at the National Cancer Center in Tokyo, of the total number of patients operated on for EGC between 2001 and 2003, only 7.6% came from mass survey, and in 77.7%, endoscopy was the initial study method [15]. It can be inferred that most EGCs in Japan are examined by opportunistic endoscopy and a smaller percentage by mass survey, which may be due, on the one hand, to population’s awareness of the risk of developing GC and on the other to the training and education of endoscopists. In Korea, in 1999, GC was included as part of a national cancer control programme, and from the beginning, people could choose between radiology and endoscopy. In both countries, when comparing both methods, endoscopic screening performance is 3 to 4 times higher, had a better early detection rate and lower cost [16,17]. Even though it is an invasive procedure, it is a one-step method, which also allows for immediate biopsy and complements tissue sample studies for investigating H. pylori, an aetiological agent in several gastric pathologies that also plays an important role in the development of GC [18,19].
Performance and EGC rate of total cancers
One of the objectives of the programme was to evaluate performance in GC detection, a fundamental factor when analysing health costs. The 2.3% (one cancer for every 43 patients studied) is higher than the mass surveys of Japan and Korea (0.1%), [20,21] and similar to a study conducted in the United Kingdom in a symptomatic population (2%) and a publication of the first 4 years of this programme (1.9%) [22,23]. Even though the performance in men is very high at 5.1% (one cancer every 20 men examined) compared to 1.2% in women (one cancer every 84), it is a datum that must be evaluated in the context of the other variables analysed. The EGC rate of 34.2% of the total GC diagnosed, can be controversial since in the untreated group, there was no confirmation of the depth of the invasion. The endoscopic differential diagnosis between AGC and EGC, in the vast majority of cases, does not present difficulties, since according to the classifications, the macroscopic characteristics are different, but there is a group called “earlylike advanced cancer” that resembles early-stage lesions, but that involves beyond the submucosa, in which it is more difficult to determine lesion depth. It has been established that the accuracy of the endoscopist in distinguishing between EGC and early-like advanced cancer, based on adequate observation of the macroscopic characteristics of the lesion, is correctly done in 83.6%, and the percentage of these macroscopic forms is low, 7.5%, compared to the total number of cancers [24]. In addition, to ensure the accuracy of the EGC rate, the causes of death and survival of 5 patients with macroscopic characteristics of EGC who refused treatment were analysed. Three of these patients died from other cancers at 15, 62, and 64 months after the initial diagnosis of EGC, one patient died from AGC at 69 months without treatment, and the last patient underwent total gastrectomy for AGC 92 months later, so that it can be affirmed that 34.2% is very close to reality and with a significant difference between men (27.6%) and women (46.6%). This EGC rate for both sexes is the highest rate published in Chile and higher than the only research programme carried out previously in our country, which reached 14.7% [25].
EGC rate in resected patients
The EGC rate in relation to the total number of resected patients, irrefutable because all have histological confirmation of the depth, in Chile has experienced a slight increase reaching 20% [26,27]. In a study of patients resected for GC in our hospital referred from various endoscopy units, which included the subgroup referred from SCC in a similar period, the early-stage rate was 16.7% [28] contrasting with 45% of EGC referred from the SCC, which would double the rate of Chilean surgical series. In a comparative study between 15 Eastern and Western countries, of patients with RO resection (no residual tumour), the early detection rates of our programme, which also included R1 and R2 patients (with micro and macroscopic residual tumour), are similar to Asian countries with mass surveys, Japan 58.3% and Korea 47.6%, and higher than other Asian (18.1%), and Western (28.2%) countries [29]. When considering only women, the early detection rate in resected patients of 54.8% is similar to Japanese and Korean multicentre surgical series of 49.7% and 57.6%, respectively [30,31].
Progression
In Japan, the rate of early detections in resected patients in the Tokyo NCC increased from 20% in the 1960s to 75% in the 2000s [32]. Since the beginning of the cancer control programme, rates in Korea have also increased. The rate increased from 28.6% in 1995 to 61% in 2014 [33,34]. The significant increase in the rates of EGC in these countries is due to the notable technological advances, training and accreditation of endoscopists, cumulative experience and knowledge of these early lesions and that they have been programmes that have lasted over time. In this series, when dividing the 17 years into 3 periods, a sustained increase in the early detection rate in women was observed, both in total cancers (36% to 54.3%) and in resected cancers (36.4% to 64.9), similar to what happened in countries with mass surveys, though not observing this increase in men. This gender disparity could be explained because in our country, public health programmes have been carried out for a long time aimed at women, sensitizing them to health problems and earlier consultation. In men, in addition to not having had this type of campaign, other factors considered risk factors for gastric cancer may also be involved in this disparity, such as low socioeconomic status, low education and high consumption of alcohol and tobacco. In a multivariate analysis of factors that influence adherence to GC screening in Korea, these three factors and a negative attitude towards preventive programmes were significantly associated with low participation in mass screening programmes [35].
Resectability
Another important objective was to determine resectability, the number of patients who could be treated at the time of diagnosis. This variable should be based on population records and form part of the evaluation in cancer screening programmes. Surgical or hospital series can show significant resectability rates but have the bias of not representing the total number of cancers in a given population and geographic area. The 68.1% resectability for both sexes of this programme is similar to a prospective hospital series in Portugal in which there were no significant changes in 3 decades despite the increase in the early detection rate from 14.5% to 20.8% and of resected stage IV from 11.8% to 27.3% [36]. Gender disparity in our programme confirms earlier consultation in women with very high resectability 78.8% vs 62.4% in men. In addition, this variable should be analysed relative to the early detection rate of resected patients since in men, a 38.4% EGC may seem adequate for the West, but among them, resectability was low, while in women, the early detection rate of 54.8% was with high resectability rate. The main cause of unresectability was the spread of the disease. These patients are referred directly to palliative care units, and there were no records in surgical series. Another cause of unresectability are patients who cannot be treated due to high surgical risk due to pre-existence of comorbidity, this group of patients has been increasing due to population ageing, and the expectation and quality of life and the risk associated with treatment must be evaluated individually. In this series, 6.5% (15 female and 7 male) were not treated for this cause. A third cause of unresectability are patients who are afraid of being diagnosed with GC and decide not to be treated or seek alternative therapies. According to our results, those who refused treatment or whose cause of nontreatment was unknown were predominantly men. The resectability rate and the causes of nontreatment can serve as a baseline for evaluation in new studies.
Survival
According to a comparative study of cancer survival worldwide, based on population registries, which included GC in 62 countries, Chile would be in the group of countries with the lowest survival, 16.4% at 5 years, without significant changes compared to previous periods [37]. The survival of the total number of cancers investigated in this programme was 38%, more than double the survival of that study, considering that there may be some differences by the statistical method used, and triples previous studies of survival, based on population records in Chile and the United Kingdom (12%) [5,6] with greater impact of the programme on survival in women 50.7% than in men 30.8%.
Survival of resected patients
The most important prognostic factors in GC are the depth of the lesion and lymph node involvement. Other factors that influence prognosis, among others, are the different treatment modalities. In this series, patients treated were managed with similar criteria following the recommendations of the clinical guidelines of the Japanese school, D2 lymphadenectomy and its subsequent modifications in early lesions (D1 + α or β) and progressively incorporating other treatment alternatives such as laparoscopic surgery and endoscopic treatments in early stages. In our hospital these techniques have been progressively developed as early lesions were increasingly detected, and the trained human resources and equipment necessary became available [38,39]. Survival in women of 69.9% is similar to a Japanese series with the highest number of resected patients, 73.7% which, like ours, included patients with endoscopic resection and R1 and R2 patients, and with similar rates of EGC [30].
Endoscopic screening in the symptomatic population of GC has additional benefits that are important to add to the costeffectiveness of the programme. One of them was the detection of other malignant lesions in the upper gastrointestinal tract or endoscopic finding, complemented with other exams allowing us to locate the primary site of the neoplastic lesion. Another benefit of screening in the symptomatic population is the diagnosis of benign lesions, prevalent pathology that involves significant health costs, many of which are related to the presence of H. pylori. The routine use of the urease test and eradication of positive cases allows healing or prolonged relief of benign pathology and at least stops the histological cascade product of H. pylori inflammation. The prevalence of H. pylori in this geographic area of the population group studied was 78%, and the most frequent benign pathology was oesophagitis in 20% and ulcerative disease in 14.5% [40].
An important factor to consider in a cancer programme is the latencies or delays that occur at different levels, one that is the responsibility of the patient, such as the time that passes from the appearance of the symptoms to the medical consultation. According to studies conducted in our country, the duration of symptoms prior to medical care exceeds 6 months in more than 40% of patients [5,41]. Other latencies are mostly the responsibility of the health system to which the patient is assigned. Proper management of the endoscopy unit shortens the time between the request for the examination and its performance, for which the inclusion of the referral sheet has been very important, which allows adequate selection in primary care and prioritization of the scheduling in the endoscopy unit according to the symptoms mentioned in the sheet.
Once cancer is suspected, the time that elapses between histological confirmation, preoperative evaluation and staging, eventual evaluation by other specialists and the decision of the treatment modality should be minimal, and requires an adequate management of the various services involved.
For an assessment of these latencies, it is essential to know the natural history of GC, these studies present difficulties and biases that should be consider. These studies were based on patients who refused or had delays in their treatment, and the evolution of the macroscopic characteristics of the lesions or doubling times was observed through endoscopy and radiological images, which provided an approximate idea for assessing the latencies in the different stages of the disease. According to these studies, a median progression of early to advanced lesions has been estimated between 34 and 44 months, and doubling times with a wide range from 1.6 to 9.5 years and in AGC the progression would be faster, from T2 to T3, 9 months and T3 to T4 3.8 months, and doubling time a range of 69 to 305 days [42-44]. It can be deduced that in both EGC and AGC, there would be fast-growing tumours and others that progress slowly, but in AGC, the progression would be faster as the tumour deepens, and the latencies in different stages of the disease have a negative impact on treatment and survival.
Limitations
One of the important limitations of this programme is that it was performed by one endoscopist; however, the training of endoscopists is currently easier due to technological advances, greater knowledge of these early lesions and online learning systems [45,46]. Recently, the development of artificial intelligence the diagnosis of early lesions would mean that less expert endoscopists or those in training would achieve results similar to those of experts [47], which would allow the establishment of early detection programmes in regions that do not have trained endoscopists.
Conclusion
An organized programme in a symptomatic population of 40 years and older has a high performance, improves early detection rates and positively impacts survival. This strategy can be replicated in regions with a high risk of developing GC through organized screening meeting certain requirements: a) involving primary care physicians and training them for adequate referral and maintaining close collaboration with them, b) adequate management of the endoscopy unit to minimize the time between the request for endoscopy and performing it, c) endoscopists trained in the detection of early lesions, d) close collaboration between pathologists and endoscopists with periodic meetings, correlating endoscopic and histological findings, e) close collaboration between endoscopists and surgeons to decide the best treatment for each patient and f) periodic evaluation of the results.
To increase participation in men and an earlier consultation, an educational campaign should be carried out to raise awareness regarding the importance of adhering to this type of screening, especially targeted at the most vulnerable population.
Declarations
Author contributions:
• Contribution Design and implementation of the project, literature research, database creation, data analysis, original and final writing (ACB). Statistical analysis, critical review of original and final working (GIN).
• Preparation of tables and figures, critical review of original and final working (LNF).
• Critical review of original work (SBV).
• Critical review of original work (ENM).
• Stimulate and facilitate new treatment modalities for EGC (ADF).
• Endoscopy-histology correlation meetings (MPA).
• Endoscopy-histology correlation meetings (ENN).
• Endoscopy-histology correlation meetings (GAB).
• Endoscopy-histology correlation meetings (P LC).
Conflicts of interest: The authors declare no conflicts of interest.
Acknowledgements: Mrs. Patricia Verdugo L, endoscopy assistant and Mrs. Margarita Arévalo C, secretary, without their help the development of the programme would not have been possible.
Funding: The authors declare that this study was not funded.
References
- Tominaga S. Primary prevention. In: Sugimura T and Sasako M, eds. Gastric Cancer. Oxford University Press 1997: 191-198.
- Departamento de Estadísticas e Información en Salud. Bases de datos Defunciones. Santiago de Chile: Ministerio de Salud. 1995-2017.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424.
- Icaza G, Núñez L, Torres-Avilés F, et al. Atlas de mortalidad en Chile, 2001- 2008. Editorial Universidad de Talca, 2013.
- Heise K, Bertran B, Andia ME, Ferreccio C. Incidence and survival of stomach cancer in a high-risk population of Chile. World J Gastroenterol. 2009; 15: 1854-62.
- Coupland V, Allum W, Blazeby J, Mendall M, Hardwick R, Linklater K et al. Incidence and survival of oesophageal and gastric cancer in England between 1998 and 2007, a population-based study. BMC Cancer. 2012; 12: 11.
- Otha H, Noguchi Y, Takagi K, Nishi M, Kajitani T and Kato Y. Early gastric carcinoma with especial reference to macroscopic classification. Cancer. 1987; 60: 1099-06.
- Sasako M, Sano T, Katai H, Maruyama K. Radical Surgery In: Sugimura T and Sasako M, eds. Gastric Cancer. Oxford University Press. 1997; 223-248.
- Maruyama M and Kimura K. Early gastric carcinoma presenting as dyspepsia. In: R Val Heatley and Peter H. Moncur, eds. Dyspepsia: The Clinical Consequences. Cambridge University Press. 2000; 175-186
- Sakita T, Oguro Y, Takasu S, Fukutomi H, Miwa T, Yoshimori M. Observations on the healing of ulcerations in Early Gastric Can-cer. Gastroenterology. 1971; 60: 835-43.
- Suvakovic Z, Bramble M G, Jones R, Wilson C, Idle N, Ryott J. Improving the detection rate of early gastric cancer requires more than open access gastroscopy: a five-year study. Gut. 1997; 41: 308-13.
- Kim JH, Kim SS, Lee JH, et al. Early Detection is Important to Reduce the Economic Burden of Gastric Cancer. J Gastric Cancer. 2018; 18: 82-89.
- Roar Johnsen. Prevalence of dyspepsia in the community. In: Val Heatley and Peter Moncur, eds. Dyspepsia: The Clinical Consequences. Cambridge University Press. 2000: 17-31.
- Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci. 2017; 108: 101-07.
- Suzuki H, Gotoda T, Sasako M and Saito D. Detection of early gastric cancer: misunderstanding the role of mass screening. Gastric Cancer. 2006; 9: 315-19.
- Tashiro A, Sano M, Kinameri K, Fujita K and Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006; 12: 4873-74.
- Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol. 2010; 16: 245-50.
- Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012; 13: 2-9.
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M et al. Helicobacter Pylori infection and development of gastric cancer. N Engl J Med. 2001; 345: 784-89.
- Oshima A. Secondary prevention: screening methods in high-incidence areas. In: Sugimura T and Sasako M, eds. Gastric Cancer. Oxford University Press. 1997: 199.
- Leung WK, Wu M, Kakugawa Y, Kim J, Yeoh K, Goh K et al. Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008; 9: 279-87.
- Hallissey MT, Allum WH, Jewkes AJ, Ellis DJ, Fielding JWJ. Early detection of gastric cancer BMJ. 1990; 301: 513-15.
- Calvo A, Pruyas M, Nilsen E, Verdugo P. Frecuency of gastric cancer in endoscopies performed in symptomatic patients at a Secondary Care Health Center. Rev Med Chile. 2001; 129: 749-55.
- Sano T, Okuyama Y, Kobori O, Shimizu T and Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990; 35: 1340-44.
- Llorens P. Gastric cancer mass survey in Chile. Semin Surg Oncol. 1991; 7: 339.
- Csendes A, Díaz JC, Musleh M, Lanzarini E, Braghetto I, Zamorano M. Early gastric cancer. A 44 years´ experience in 304 patients. Rev Chi Cir. 2015; 67: 175-80.
- García C, Benavides C, Apablaza S et al. Surgical treatment of gastric cancer: results in 423 cases Rev Méd Chile. 2007; 135: 687-95.
- Norero E, Vega E, Díaz C, Cavada G, Ceroni M, Martínez C et al. Improvement in postoperative mortality in elective gastrectomy for gastric cancer: Analysis of predictive factors in 1066 patients from a single center Eur J Surg Oncol. 2017; 43: 1330-6.
- Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging Project. Gastric Cancer. 2017; 20: 217-25.
- Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I et al. Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007) Gastric Cancer. 2018; 21: 144-54.
- Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011; 11: 69-77.
- Yamada M, Oda I, Taniguchi H and Kushima R. Chronological trend in clinicopathological characteristics of gastric cancer. Japanese Journal of Clinical Medicine. 2012; 70: 1681-85.
- Information Committee of the Korean Gastric Cancer Association. 2004 Nationwide Gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007; 7: 47-54.
- Korean Gastric Cancer Association. Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016; 16: 131-40.
- Kwon YM, Lim HT, Lee K, Cho B L, Park MS, Son KY et al. Factors associated with use of gastric cancer screening services in Korea. World J Gastroenterol. 2009; 15: 3653-9.
- Faria G R, Pinto-de-Sousa J, Preto J R, Sousa H S, Barbosa J A, Costa-Maia J. Three decades of clinical-pathological trends in gastric cancer: Prospective data from a Portuguese hospital. Int J Surg. 2013; 11: 472-6.
- Allemani1 C, Matsuda T, Di Carlo V, Harewood R, Spika D, Wang X et al. Global surveillance of trends in cancer survival: analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers during 2000–2014 from 322 population-based registries in 71 countries (CONCORD-3). Lancet. 2018; 391: 1023–75.
- Galindo J, Rodríguez J, Norero E, Aguayo G, Pruyas M, Nilsen E, et al. Endoscopic submucosal dissection for early gastric cancer. Rev Chil Cir. 2015; 67: 590-8.
- Norero E, Báez S, Briceño E, Martínez C, Ceroni M, Escalona A et al. Totally laparoscopic gastrectomy for the treatment of gastric tumors. Rev Med Chile. 2015; 143: 281-8.
- Ortega JP, Espino A, Calvo A, Verdugo P, Pruyas M, Nilsen E et al. Helicobacter pylori infection in symptomatic patients with benign gastroduodenal diseases. Analysis of 5.664 cases. Rev Med Chil. 2010; 138: 529-35.
- Müller B, De La Fuente H, Barajas O, Cardemil B, Vila T, Mordojovich E et al. Registry of gastric cancer evaluation in Chile (REGATE): Basal clinical features of 523 patients. Rev Chi Cir. 2011; 63: 147-53.
- Oh SY, Lee JH, Lee HJ, Kim TH, Hu YJ, Ahn HS et al. Natural History of Gastric Cancer: Observational Study of Gastric Cancer Patients Not Treated During Follow-Up. Ann Surg Oncol. 2019; 26: 2905-11.
- Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000; 47: 618-21.
- Kohli Y, Kawai K, Fujita S. Analytical Studies on Growth of Human Gastric Cancer. J Clin Gastroenterol. 1981; 3: 129-33.
- Gotoda T, Uedo N, Yoshinaga S, Tanuma T, Morita Y, Doyama H et al. Basic principles and practice of gastric cancer screening using high-definition white-light gastroscopy: Eyes can only see what the brain knows. Dig Endosc 2016; 28: 2-15.
- Yao K, Uedo N, Muto M, and Ishikawa H. Development of an e-learning system for teaching endoscopists how to diagnose early gastric cancer: basic principles for improving early detection. Gastric Cancer. 2017; 20: 528-38.
- Luo H, Xu G, Li Ch, He L, Luo L,Wang Z et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicenter, case-control, diagnostic study. Lancet Oncol. 2019; 20: 1645-54.