Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Quality of life temporarily improved in patients in whom the diagnosis chronic mesenteric ischemia wasn’t confirmed after multidisciplinary evaluation in a tertiary referral centre
FM Metz1*; JTM Blauw1,2; M Brusse-Keizer3,4; JJ Kolkman5,6,7; RH Geelkerken1,5,8
1 Department of Vascular Surgery, Medisch Spectrum Twente, Enschede, the Netherlands.
2 Department of Surgery, Amsterdam University Medical Centre, Amsterdam, the Netherlands.
3 Medical School Twente, Medisch Spectrum Twente, Enschede, the Netherlands.
4 Health Technology and Services Research, University of Twente, Enschede, the Netherlands.
5 Dutch Expert Centre for Gastrointestinal Ischemia, Enschede, the Netherlands.
6 Department of Gastroenterology, Medisch Spectrum Twente, Enschede, the Netherlands.
7 Department of Gastroenterology, University Medical Centre Groningen, Groningen, the Netherlands.
8 Multi-Modality Medical Imaging group, TechMed Centre, University of Twente, Enschede, the Netherlands.
*Corresponding Author : Metz FM
Department of Vascular Surgery, Medisch
Spectrum Twente PO Box 50.000 7500 KA
Enschede, The Netherlands.
Tel +31-534872000; Email: Flores.metz@mst.nl
Received : Dec 04, 2021
Accepted : Jan 13, 2022
Published : Jan 20, 2022
Archived : www.jjgastro.com
Copyright : © Metz FM (2022).
Abstract
Objectives: Chronic Mesenteric Ischemia (CMI) is a disease in which abdominal symptoms are caused by insufficient mesenteric blood supply. Treatment results in improved quality of life (QoL). To put these results into perspective, the QoL of patients with symptoms potentially complying with CMI but without confirmation of the diagnosis was studied from six months up to four years.
Methods: Between May and July 2020 follow-up questionnaires were sent to 144 patients that were suspected of CMI but in whom the diagnosis was not confirmed after a thorough multidisciplinary evaluation in a CMI expert centre. The baseline QoL was measured at first presentation. Three cohorts were included: 50 patients with a follow-up of six months, 45 patients with a follow-up of two years, and 49 patients with a follow-up of four years were invited to participate. The QoL was measured on a 100 points Visual Analogue Scale (VAS). A minimal clinically important difference of 7.5 was used as non-inferiority threshold.
Results: The response rates were 34/50 (68%), 33/45 (73%), and 34/49 (69%). QoL improved in the six months group, with a mean change of 19 in VAS score (95% CI 11-27), in which baseline QoL was inferior to the QoL at follow-up (lower bound 95% CI above >7.5 threshold). The change in QoL was inconclusive in the other two groups, respectively 15 (95% CI 6-24) and 3 (95% CI -6-13). Furthermore, there was no significant change in QoL between patients without mesenteric stenosis and with one or two vessel stenosis (P=0.36) and between patients with occlusive stenosis and anatomic Median Arcuate Ligament Syndrome (MALS) (P=0.53).
Conclusion: The QoL of patients suspected for CMI was clinically significantly improved after six months without additional treatment. However, this improvement faded completely after four years.
Keywords: quality of Life; chronic mesenteric ischemia; mesenteric stenosis; revascularisation; sham; follow-up.
Abbreviations: 95% CI: 95% Confidence Interval; CMI: Chronic Mesenteric Ischemia; CTA: Computerized Tomographic Angiography; IQR: Interquartile Range; MALS: Median Arcuate Ligament Syndrome; MCID: Minimal Clinically Important Difference; MRA: Magnetic Resonance Angiography; QoL: Quality of Life; VAS: Visual Analogue Scale.
Citation: Metz FM, Blauw JTM, Brusse-Keizer M, Kolkman JJ, Geelkerken RH. Quality of life temporarily improved in patients in whom the diagnosis chronic mesenteric ischemia wasn’t confirmed after multidisciplinary evaluation in a tertiary referral centre. Japanese J Gastroenterol Res. 2022; 2(1): 1052.
Introduction
Chronic Mesenteric Ischemia (CMI) has been defined as insufficient mesenteric blood supply causing abdominal symptoms for at least three months [1]. Diagnosing CMI is challenging by the highly variable abdominal symptoms [2] and the high incidence of asymptomatic mesenteric artery stenosis (6 to 29% of general population) [3-11]. Also, CMI is not rare with a reported incidence up to 9.3 per 100,000 people [12]. Symptoms and anatomy of patients suspected of CMI should be evaluated in a multidisciplinary team, consisting of gastroenterologists, vascular surgeons, and radiologists who have experience with this pathology [1,13]. The multidisciplinary team either confirms the suspicion of CMI and patients are treated accordingly, or rejects the suspicion of CMI and patients are referred back to their primary physician. Although sustainable relief of abdominal symptoms after successful mesenteric artery revascularisation and thus restoring Quality of Life (QoL) is the primary goal of treatment, only three studies have focused on the QoL (after mesenteric revascularisation) [14-16]. Two of these studies demonstrated a significantly improved QoL after revascularisation treatment suggesting that the treatments were successful [14,15]. The third study showed a significantly higher QoL in patients where the symptoms had disappeared compared to patients with persisting symptoms after surgical treatment for median arcuate ligament syndrome (MALS) [16], suggesting that the symptoms decreased the experienced QoL. To put the results of these studies into perspective, this comparison study was performed in patients with symptoms potentially complying with CMI that were not treated. The present study aims to assess the QoL evolution over short-, mid-, and long-term follow-up for patients in whom a CMI diagnosis was rejected. We hypothesized that the QoL of these patients would not improve because the patients did not receive CMI treatment.
Methods
Study population
All patients suspected of CMI referred to the Medisch Spectrum Twente (MST), a tertiary referral centre for mesenteric ischemia, were included in a standardized digital mesenteric research database since 2014. The following parameters were registered: medical history, present symptoms, QoL measurement and visualization of mesenteric anatomy. The inclusion and exclusion criteria for the current study are listed in (Table 1). The referral letter and accompanying computed tomography angiography (CTA) or magnetic resonance angiography (MRA) of each referred patient were assessed “on paper” by the mesenteric ischemia group (MIG), a multidisciplinary team consisting of gastroenterologists, vascular surgeons and radiologists, to decide whether CMI was suspected based on symptoms CMI and/or significant mesenteric artery stenosis. In case of a suspicion of CMI, the patients were independently evaluated by a gastroenterologist and a vascular surgeon in the outpatient clinic. The findings were discussed in the MIG which then decided whether the diagnosis CMI should be confirmed (patients received a proposal for treatment) or rejected (patients were referred back to their primary physician).
For the current study three patient groups of the MST digital mesenteric research database were identified based on the duration of follow-up: group I) a cohort with a six months follow-up (multidisciplinary evaluation between June 2019 and January 2020), group II) a cohort with a two years follow-up (multidisciplinary evaluation between January and July 2018), and group III) a cohort with a four years follow-up (multidisciplinary evaluation between January and July 2016). Eligible patients were contacted by phone and email and asked to complete a second survey to collect their present QoL. Non-responders were approached to a maximum of five times.
Table 1: Inclusion and exclusion criteria.
Inclusion criteria |
Exclusion criteria |
- Suspicion of mesenteric ischemia based on paper assessment by the multidisciplinary team |
- Not fulfilling the inclusion criteria |
Outcome measures
QoL was measured using a single-item self-rating 20 cm VAS (Visual Analogue Scale), marked from 0 (worst imaginable quality of life) to 100 (best imaginable quality of life). This is a valid and reliable instrument for global QoL, and used for example in patients with Inflammatory Bowel Disease (IBD) [17]. A validated survey for CMI is not available. The baseline and follow-up QoL were measured with the same instrument, the patients were not reminded of their previous score. To decide whether the follow-up QoL was significantly different compared to their baseline QoL, the Minimal Clinically Important Difference (MCID) was used. Because an MCID for CMI on the VAS QoL scale has not been determined, we used an MCID of 7.5 based on scores calculated for Chronic Obstructive Pulmonary Disease (COPD), stroke and obesity patients [18-20].
Statistical analysis
A sample size of 34 patients per group was calculated with a one-sided power analysis with alpha 2.5%, an assumed difference of 0, a Standard Deviation of 15 between the QoL at follow-up and the QoL at intake and a non-inferiority threshold of 7.5 to achieve a power of 80%. Continuous variables were displayed as means (standard deviation) or median (interquartile range, IQR) for respectively parametric and non-parametric. Categorical variables were displayed as numbers (percentages). Normally distributed continuous variables were compared between the different groups with an ANOVA test with post hoc Tukey’s Honestly Significant Difference test. Not normally distributed continuous variables were compared with a KruskalWallis test with post hoc Bonferroni Holm corrected MannWhitney U test. Categorical variables were compared with a Chi Square test or in case of small sample sizes with a Fisher Exact test. Paired continuous variables were compared with a paired samples T-test. The α-level was set on 0.05. To prove non-inferiority, the 95% confidence interval (95% CI) of the difference in QoL between baseline and follow-up should not exceed the defined non-inferiority threshold of 7.5. The change in QoL (follow-up QoL minus baseline QoL) was analysed for three groups based on follow-up period: group I, group II and group III. The mean change in QoL was also analysed for two groups based on the number of mesenteric artery stenosis: group A) the patients with zero vessel stenosis, and group B) the patients with one or two vessel stenosis. Group B was then subdivided based on type of significant stenosis: group B1) the patients with a significant one or two vessel atherosclerotic stenosis, and group B2) the patients with anatomic median arcuate ligament syndrome (MALS). A multivariate analysis was performed to investigate whether these results of comparison A/B were biased by a difference in follow-up period between the groups. Furthermore, to detect a possible selection bias the baseline characteristics between the responding patients, non-responding patients and the patients whose QoL VAS scale at intake was not registered because of an administrative error were compared.
The power calculation was performed with PASS 11.0 (NCSS, LLC. Kaysville, Utah, USA) and the statistical analyses were performed using SPSS Statistics version 27.0 (IBM Corporation, Armonk, NY, USA). This study was exempted from approval from the Institutional Review Board.
Results
Patient population
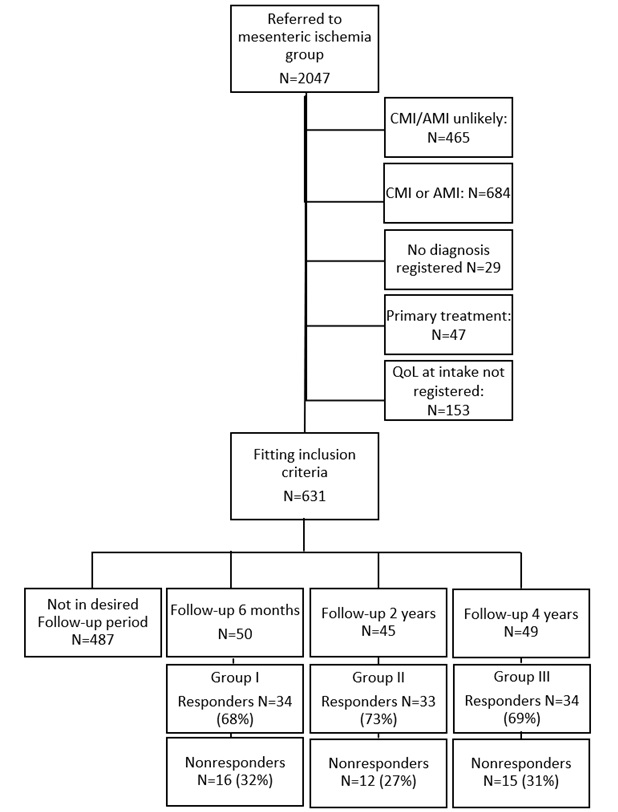
Within the inclusion period, 631 patients registered in the database met the inclusion criteria. From these 631 patients, 144 were eligible to participate in the study: group I n=50, group II n=45, and group III n=49. Of these patients, 43 patients were excluded, because they did not respond after five approaches (n=30), refused participation (n=10) or passed away (n=3). This resulted in a final inclusion of 34 patients in group I, 33 patients in group II and 34 in group III, see (Figure 1).
Table 2: Patient characteristics in groups based on follow-up period.
|
Group I |
Group II |
Group III |
p-value |
Gender, women, n (%) |
23 (68) |
28 (85) |
23 (68) |
0.19# |
Age at intake (IQR) |
56 (32-69) |
65 (54-71) |
57 (48-65) |
0.14## |
Number of mesenteric stenosis (%) |
11 (32) |
12 (36) |
23 (68) |
<0.01### |
Secondary treatment, n(%) |
1 (3) |
|
|
|
# Chi Square test
## Kruskal-Wallis test
### Fisher Exact test
Supplementary Table 1: Patient characteristics of the excluded patients.
|
Responders (n=101) |
Non-responders (n=43) |
QoL at intake not registered (n=153) |
P
|
Gender, women, n (%) |
74 (73) |
31 (72) |
102 (67) |
0.50# |
Age at intake (IQR) |
60 (43-70) |
56 (45-69) |
60 (40-73) |
0.54## |
Number of mesenteric stenosis (%) |
46 (46) |
13 (30) |
75 (49) |
0.13### |
Median QoL at intake (IQR) |
45 (25-65) |
28 (10-67) |
- |
0.09## |
# Chi Square test
## Kruskal-Wallis test
### Fisher Exact test
(Table II) shows the baseline characteristics between the different follow-up groups. There were significantly more zero vessel stenosis in group III compared to groups I and II (P<0.01).
Supplemental Table I reports the basic characteristics of the responding, the non-responding and the patients whose QoL VAS scale at intake was not registered. There was no significant difference in gender, age at intake, number of mesenteric stenosis and QoL at intake between these groups.
Change in QoL
Group I showed a significantly improved QoL with an increase of 19 points on the VAS scale (95% CI 11-27), in which baseline QoL was inferior to the QoL at follow-up (lower bound 95%CI above >7.5 threshold). The change in QoL was inconclusive in the other two groups, Table III. The difference in change in QoL between the three follow-up groups was significant (P=0.04) and originated from the significant difference in change in QoL between group I and group III of 16 points (P=0.04).
Furthermore, there was no significant change in QoL between patients without mesenteric stenosis (Group A) and with one or two vessel stenosis (Group B) in a follow-up period between six months and four years (P=0.36) (Table IV). Within group B, there was no significant difference in change in QoL between patients with atherosclerotic mesenteric artery stenosis (B1) and anatomic MALS (Group B2) (P=0.53) (Supplemental Table II). Due to a difference in follow-up time between patients with and without mesenteric stenosis, a multivariate linear regression analysis was performed to correct for this potential confounder. After correction for follow-up, the change in QoL between group A and B (P=0.07) and between group B1 and B2 was still not significant (P=0.67).
Table 3: Quality of Life (QoL) before and after follow-up in groups based on follow-up period.
Patients |
Group I |
Group II |
Group III |
P |
Mean change QoL * (95% CI) |
19 (11-27) |
15 (6-24) |
3 (-6-13) |
0.04# |
Median QoL intake (IQR) |
35 (27-60) |
45 (20-68) |
50 (30-66) |
0.51## |
Median QoL follow-up (IQR) |
68 (47-79) |
66 (45-80) |
56 (44-68) |
0.08## |
* Follow-up QoL minus baseline QoL
# ANOVA test
## Kruskal-Wallis test
Table 4: Quality of Life (QoL) before and after follow-up in groups based on number of mesenteric stenosis.
Patients |
Group A |
Group A |
P |
|
Gender, women, n (%) |
|
34 (74) |
40 (73) |
0.89# |
Age at intake (IQR) |
62 (44-67) |
56 (41-70) |
0.67## |
|
Follow-up Group (%) |
I (6 months) |
11 (24) |
23 (42) |
<0.01# |
|
II (2 years) |
12 (26) |
21 (38) |
|
|
III (4 years) |
23 (50) |
11 (20) |
|
Mean change QoL * (95% CI) |
14 (6-23) |
10 (3-17) |
0.36### |
|
QoL intake (IQR) |
|
45 (30-61) |
47 (20-68) |
1.00## |
QoL follow-up (IQR) |
64 (46-80) |
60 (40-73) |
0.39## |
|
* Follow-up QoL minus baseline QoL
# Chi Square test
## Mann-Whitney test
####ANOVA test
Supplemental Table 2: Quality of Life (QoL) before and after follow-up in groups based on type of significant stenosis
Patients |
Group B1 |
Group B2 |
P |
|
Mean change QoL * (95% CI) |
|
8 (-5-20) |
12 (4-20) |
0.53# |
Median QoL intake (IQR) |
|
38 (18-69) |
50 (20-77) |
0.66## |
Median QoL follow-up (IQR) |
53 (36-67) |
66 (45-75) |
0.26## |
|
* Follow-up QoL minus baseline QoL
# ANOVA test
## Mann-Whitney test
Discussion
This study is the first to evaluate the evolution of QoL in patients in which the diagnosis of CMI was rejected after multidisciplinary evaluation. The hypothesis that the follow-up QoL would be non-inferior compared to the baseline QoL, was rejected for the six months follow-up group. The QoL of these patients improved clinically significantly with a mean increase of 19 on a 100 points VAS scale. After two years, the QoL was numerically lower and baseline QoL was no longer inferior to Qol at follow-up, the effect completely faded after four years. The short-term increase in QoL may have been an effect of the attention and thorough evaluation at the expert centre for mesenteric ischemia. In the present study there was no significant difference in QoL improvement between patients with zero vessel stenosis and one or two vessel stenosis and between patients with coeliac artery compression and with one or two vessel atherosclerotic stenosis.
This study was performed to put the results of three studies on QoL after revascularization in CMI patients into perspective. Blauw et. al [15] investigated QoL after revascularisation in CMI patients using VAS scores of 31 patients that were measured between six months and three years after revascularisation treatment which improved with a mean change of 16 compared to intake measurement. Skelly et al. [14] investigated the Health Related Quality of Life (HRQoL) in 51 MALS patients before and six months after surgery which improved with a mean change of 12. Pather et al. [16] investigated the Gastrointestinal Quality of Life Index (GIQLI) of 46 MALS patients after coeliac artery release with a mean follow up of 8 years after surgery, with a significantly higher mean QoL of 80 GIQLI in 30 patients where the symptoms had disappeared compared to 53 in 16 patients with persisting symptoms. Since our patients with symptoms potentially complying with CMI showed a comparable shortterm increase in QoL without treatment, the improvement in QoL in studies with follow-up of six months [14,15], could be (partially) caused by another effect then treatment. The short-term increase in QoL seemed temporarily and disappeared completely after four years, the appropriate follow-up period to detect real changes in QoL may even be four years. Attention and recognition for the disabling symptoms influences the patient’s perceived QoL; 151 hospitalised older adults demonstrated that 70% of the participants changed their VAS score more than 5 points after a detailed explanation of their extremely good or poor health status [21]. Also, patients with Irritable Bowel Syndrome (IBS), showed improved QoL caused by psychological therapy [22-25]. Consequently, the improvement in QoL in the first months after a thorough analysis of the symptoms as observed in the present study may occur without an objective change in underlying health state and should be interpreted with caution. In other words, this indicates that for assessment of the QoL improvement of an intervention, a six months period may be too short, or should be corrected for the effect in patients that did not receive treatment.
This study is thus far the largest study investigating the development of the QoL in a representative population of patients with symptoms potentially complying with CMI but after multidisciplinary evaluation ultimately rejected diagnosis of CMI with a follow-up period varying from six months to four years. The evaluation took place in the Dutch Expert Centre for Gastrointestinal Ischemia. During the study period the procedure of the evaluation did not change. The transversal study design offers the opportunity to compare the follow-up score to the intake measurement of the same patient.
A limitation of the transversal study design is a potential difference in characteristics and baseline QoL between the three follow-up groups. However, the only significant difference that was found was that the four years follow-up group (group III) comprised of more patients with zero vessel stenosis whereas the other two groups comprised of more patients with one vessel stenosis. Nevertheless, no significant difference in QoL change was shown between the patients with zero vessel stenosis and one or two vessel stenosis in current research. Another limitation of the study is a potential selection bias caused by the group of patients in which the intake VAS score was not registered, and the non-responders. However, the basic characteristics of these patients were not significantly different from the characteristics of the responders (data in supplemental table I), making it unlikely that the selection bias was actually present.
Conclusion
There is a clinical and statistically significant QoL improvement in patients with chronic abdominal pain, but not diagnosed with CMI, within the first six months after an intensive diagnostic trajectory without any therapeutic intervention. However, this QoL improvement completely faded after four years follow-up. Hence, attention and extensive evaluation for the unexplained complaints at an expert centre may lead to short-term improvement of experienced QoL. This indicates that for assessment of the QoL improvement of an intervention, the appropriate follow-up period to detect real changes in QoL may be four years.
References
- Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, et al. Editor’s Choice – Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017; 53: 460-510.
- van Dijk LJD, Noord D van, de Vries AC, Kolkman JJ, Geelkerken RH, Verhagen HJM, et al. Clinical management of chronic mesenteric ischemia. United Eur Gastroenterol J. 2019; 7: 179-88.
- Derrick JR, Pollard HS, Moore RM. The pattern of arteriosclerotic narrowing of the celiac and superior mesenteric arteries. Ann Surg. 1959; 149: 684-9.
- Croft RJ, Menon GP, Marston A. Does ‘intestinal angina’ exist? A critical study of obstructed visceral arteries. Br J Surg. 1981; 68: 316-8.
- Järvinen O, Laurikka J, Sisto T, Salenius JP, Tarkka MR. Atherosclerosis of the visceral arteries. Vasa. 1995; 24: 9-14.
- Bron KM, Redman HC. Splanchnic Artery Stenosis and Occlusion. Radiology. 1969; 92: 323-8.
- Mensink PBF, Moons LMG, Kuipers EJ. Chronic gastrointestinal ischaemia: shifting paradigms. Gut. 2011; 60: 722-37.
- Glockner JF. Incidental findings on renal MR angiography. Am J Roentgenol. 2007; 189: 693-700.
- Hansen KJ, Wilson DB, Craven TE, Pearce JD, English WP, Edwards MS, et al. Mesenteric artery disease in the elderly. J Vasc Surg. 2004; 40: 45-52.
- Park CM, Chung JW, Kim HB, Shin SJ, Park JH. Celiac Axis Stenosis: Incidence and Etiologies in Asymptomatic Individuals. Korean J Radiol. 2001; 2: 8.
- Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. Am J Roentgenol. 1993; 161: 985-8.
- Terlouw LG, Verbeten M, van Noord D, Brusse-Keizer M, Beumer RR, Geelkerken RH, et al. The Incidence of Chronic Mesenteric Ischemia in the Well-Defined Region of a Dutch Mesenteric Ischemia Expert Center. Clin Transl Gastroenterol. 2020; 11: e00200.
- Terlouw LG, Moelker A, Abrahamsen J, Acosta S, Bakker OJ, Baumgartner I, et al. European guidelines on chronic mesenteric ischaemia – joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepa. United Eur Gastroenterol J. 2020; 8: 371-95.
- Skelly CL, Stiles-Shields C, Mak GZ, Speaker CR, Lorenz J, Anitescu M, et al. The impact of psychiatric comorbidities on patientreported surgical outcomes in adults treated for the median arcuate ligament syndrome. J Vasc Surg. 2018; 68: 1414-21.
- Blauw JTM, Pastoors HAM, Brusse-Keizer M, Beuk RJ, Kolkman JJ, Geelkerken RH, et al. The Impact of Revascularisation on Quality of Life in Chronic Mesenteric Ischemia. Can J Gastroenterol Hepatol. 2019: 1-7.
- Pather K, Kärkkäinen JM, Tenorio ER, Bower TC, Kalra M, DeMartino R, et al. Long-term symptom improvement and healthrelated quality of life after operative management of median arcuate ligament syndrome. J Vasc Surg [Internet]. 2020; S0741- 5214: 32480-0.
- Stark RG, Reitmeir P, Leidl R, König H-H. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010; 16: 42-51.
- Zanini A, Aiello M, Adamo D, Casale S, Cherubino F, Della Patrona S, et al. Estimation of Minimal Clinically Important Difference in EQ-5D Visual Analog Scale Score After Pulmonary Rehabilitation in Subjects With COPD. Respir Care. 2015; 60: 88-95.
- Chen P, Lin K-C, Liing R-J, Wu C-Y, Chen C-L, Chang K-C. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual Life Res. 2016; 25: 1585-96.
- Warkentin LM, Majumdar SR, Johnson JA, Agborsangaya CB, Rueda-Clausen CF, Sharma AM, et al. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: two-year prospective cohort study. BMC Med. 2014; 12: 175.
- McPhail S, Beller E, Haines T. Reference bias: presentation of extreme health states prior to EQ-VAS improves health-related quality of life scores. A randomised crossover trial. Health Qual Life Outcomes. 2010; 8: 146.
- Altayar O, Sharma V, Prokop LJ, Sood A, Murad MH. Psychological Therapies in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract. 2015; 2015: 1-19.
- Basnayake C, Kamm MA, Salzberg MR, Wilson‐O’Brien A, Stanley A, Thompson AJ. Delivery of care for functional gastrointestinal disorders: A systematic review. J Gastroenterol Hepatol [Internet]. 2020; 35: 204-10.
- Kruimel J, Leue C, Winkens B, Marcus D, Schoon S, Dellink R, et al. Integrated medical–psychiatric outpatient care in functional gastrointestinal disorders improves outcome. Eur J Gastroenterol Hepatol. 2015; 27: 721-7.
- Kinsinger SW, Ballou S, Keefer L. Snapshot of an integrated psychosocial gastroenterology service. World J Gastroenterol. 2015; 21: 1 893.