Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 2
Antiulcerogenic effect of Aristolochia argentina Griseb. (Aristolochiaeceae) in rat
Paredes Jésica D1*; Chaves Maximiliano E2; Mohamed Fabián H2; Pelzer Lilian E1; Maria Alejandra O1; Wendel Graciela H1
1 Laboratory of Pharmacology, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, Argentina.
2 Laboratory of Histology, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, Argentina.
*Corresponding Author : Paredes Jésica D
Laboratory of Pharmacology, Faculty of Chemistry,
Biochemistry and Pharmacy, National University of
San Luis, Argentina.
Email: jdparedes@unsl.edu.ar
Received : Dec 01, 2021
Accepted : Jan 10, 2022
Published : Jan 17, 2022
Archived : www.jjgastro.com
Copyright : © Paredes Jésica D (2021).
Abstract
Introduction: The roots of Aristolochia argentina Griseb. (Aristolochiaceae) are used in folk medicine for the treatment of colitis, diarrhea and hemorrhoids.
Methods: The aqueous extract of A. argentina was concentrated and lyophilized (AALE) to preserve it. AALE was studied in rats for its ability to protect gastroduodenal mucosa from the injuries caused by ulcerogenic agents. The cytoprotective mechanisms were also evaluated.
Discussion: AALE exhibited a significant protective effect against ulcerogenic agents (EtOH, p<0.001; HCI, p<0.01; NaOH, p<0.05; HCl/ EtOH, p<0.01). The protective effect of AALE on lesions induced for ethanol was confirmed by gastric histological examination. Indomethacin (p<0.001) and Nω-nitro-L -arginine (p<0.001) were able to significantly counteract the antiulcerogenic activity of AALE against ethanol injury. AALE produced significant antiulcerogenic activity in rats in experimentally induced gastric ulcers.
Conclusion: The participation of the prostaglandins and nitric oxide seem to be involved in the antiulcerogenic activity of AALE. Nevertheless, this activity does not seem to be related with participation of endogenous sulfhydryls.
Keywords: Aristolochia argentina; antiulcerogenic activity; gastric ulcers; ethanol.
Abbreviations: AALE: Aristolochia argentina Lyophilized Extract; ANMAT: Administración Nacional de Medicamentos, Alimentos y Tecnología Médica; CICUA: Animal Care and Use Institutional Committee; CO2 : Carbonic Anhydride; COX: Cyclooxygenase; EGF: Epidermal Growth Factor; EtOH: Ethanol; FRAP: Ferric Reducing Antioxidant Power; H&E: Haematoxylin-Eosine; HCl/EtOH: Hydrochloric Acid/ Ethanol; HCl: Hydrochloric Acid; L-NNA: Nω-Nitro-L-Arginine; NaOH: Sodium Hydroxide; NEM: NEthylmaleimide; NO: Nitric oxide; NOS: NO Synthase; NPSH: Non-Protein Sulfhydril Groups; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; p.o.: per os; PDEGF: PlateletDerived Growth Factor; PG: Prostaglandin; E2: Prostaglandins E2 ; ROS: Reactive Oxygen Species; VEGF: Vascular Endothelial Growth Factor.
Citation: Paredes Jésica D, Maximiliano EC, Fabián HM, Lilian EP, Alejandra OM, et al. Antiulcerogenic effect of Aristolochia argentina Griseb. (Aristolochiaeceae) in rat. Japanese J Gastroenterol Res. 2022; 2(1): 1051.
Introduction
The gastric mucosa is continuously exposed to multiple factors and harmful substances that may trigger the onset of gastric ulcers, this being the most prevalent gastrointestinal disease worldwide [1]. The exploration for a different treatment has motivated investigation on medicinal plants. The pathophysiology involves an imbalance between aggressive factors (ethanol (EtOH), reactive oxygen species (ROS) acid-pepsin secretion, alcoholic beverages, use non-steroidal anti-inflammatory drugs (NSAIDs), tobacco and Helicobacter pylori infection) and defensive factors (mucus secretion, blood flow, prostaglandin (PG), bicarbonate, nitric oxide (NO), non-protein sulfhydril groups (NP-SH) and epidermal growth factors) [1,2]. In the expectation of opposing various injurious agents, the gastric tissue has several defense mechanisms, such as the presence of the gastric mucosa, which undergoes a change every two to six days. This is maintained by the balance between proliferation and apoptosis in the gastric epithelial cells, essential to homeostasis, the morphology and physiology of the gastric mucus barrier [3]. The studies of plants used by folk in the treatment of peptic ulcer are viable alternatives to currently available drugs.
Aristolochia argentina Griseb. (Aristolochiaceae) is popularly known as “charrúa”, “charruga”, “mil hombres”, “patito”, “buche de pavo”. The dried aerial parts are used to insecticide, antioxidant, antibacterial, and antifungal activity and root as used anti-ulcerogenic [4]. The infusions and tinctures are used mainly for the treatment of colitis, diarrhea and haemorrhoids [5]. Previous studies shown that extracts from roots of A. argentina exhibit pharmacological antidiarrheal activity and did not show any toxic effect on acute toxicity evaluation test in mice [6]. Phytochemical results of aqueous root extracts revealed presence of the carbohydrates, flavonoids, tannins, saponins and anthraquinones [6].
Priestap et al. (2003) [5], in previous investigations of A. argentina, revealed the presence of aristolochic acids, alkaloid (allantoine) and argentilactone (a 5-hydroxy acid lactone). Plants are some of the most attractive sources of new drugs, and some have been shown be effective for the treatment of gastroduodenal ulcer with minimum side effects. Plants with traditional ethnomedicinal uses in ulcerative lesions management thus need to be screened for potential antiulcer activity. The objective of this study was to evaluate the anti-ulcerogenic activity of A. argentina and possible mechanisms of action, in experimental models of gastric ulcers in rats.
Material and methods
Plant material
Roots of A. argentina were collected in the San Luis province, Ayacucho Department, Lujan locality. The botanical identification of species was made through the application of classical taxonomic methods and certified by Dr. Luis Del Vitto. The voucher specimen was deposited in the Herbarium of the National University of San Luis, San Luis, Argentina, under the registry No. 9258. The collection of plant material was approved by the chief of “Biodiversity” program, Ministry Environment of the government of San Luis province (Resolution Nº 588-PBD2014).
Extraction procedure
The roots collected were cleaned, selected and desiccated, and then the dry material was mechanically milled. Infusion of roots of the plant at 10% was prepared according to VI Ed Argentine National Pharmacopoeia. The plant material was separated by filtration and the aqueous extract was concentrated and lyophilized to preserve it.
Animals
Six to eight adult male Wistar rats (150–180 g) were used in each group. They were housed in standard environmental conditions and fed with rodent diet and water ad libitum. The animals were housed at a room temperature of 24 ± 1°C with 12 h light/dark cycle. The animals were randomly assigned to different groups and a period of 4 days was allowed to adapt to each experiment. All experiments were in compliance with the ANMAT (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica) No 6344/96 for animal care guidelines. Experimental protocols were approved by Animal Care and Use Institutional Committe (CICUA) of Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis (approval numbers, F-80/11, F-104/12, F-103/12, F-85/11, F-90/12, F-93/12, Res. Nº 1498/13). Rats were euthanized in a presaturated CO2 chamber.
Pharmacological studies
The A. argentina lyophilized extract (AALE) was used for the pharmacological study.
Effects of AALE on gastric ulcers induced by EtOH, HCI, NaOH and HCl/EtOH: One of the following ulcerogenic agents was given per os (p.o.) in a volume of 1 ml/200 g: absolute ethanol (EtOH), 0.6 N Hydrochloric acid (HCl), 0.2 N Sodium hydroxide (NaOH) and 0.3 M/60% Hydrochloric Acid/ Ethanol (HCl/EtOH) [7]. AALE (125, 250 and 500 mg/kg, p.o.) was administered 1 h before agents. Control rats were treated with an equal volume of the corresponding vehicle. In positive control groups, animals were treated with carbenoxolone (250 mg/ kg, p.o.). Rats were euthanized 1 h after agents. The stomachs were removed and inspected for lesions in the glandular portion. A scanner examined the specimens and the scanned image was analyzed by using a program developed by the National Institutes of Health (Image J 1.46r). In brief, the scanned image was converted to gray scale, the total corpus mucosal area was recorded, the unlesioned area was subtracted and the gastric lesion area was measured (12 pixels = 1 mm) according to the method described by Khan (2004) [8].
Histological examination of gastric ulcers induced by EtOH: The stomachs were surgically removed and sliced into 8–10 mm crosssectional slabs. For light microscopic evaluation, the samples were fixed in Bouin’s solution, dehydrated in an increasing ethanol series, and embedded in paraffin. Sections, 3-4 μm thick, were stained with haematoxylin-eosine (H&E). After, the slides were examined under a light microscope (Olympus BX-40, PA) interfaced with a computer hosting the image processing and recording system. The images were captured by a digital camera (Sony SSCDC5OA).
Effects of AALE on gastric ulcers induced by acetylsalicylic acid, phenylbutazone and reserpine: NSAID-induced gastric ulcers were produced using acetylsalicylic acid and phenylbutazone. AALE (500 mg/kg) was administered p.o., 30 min before the ulcerogenic agents. Acetylsalicylic acid was suspended in 0.5 % carboxymethylcellulose in water and administered in the dose of 200 mg/kg (p.o.) to 40 h fasted rats according to the method of Hemmati (1973) [9]. Four hours after aspirin administration the animals were euthanized. Phenylbutazoneinduced gastric lesions were produced following the method of Galil (1968) [10]. A single dose of phenylbutazone (200 mg/ kg) was intraperitoneally (i.p.) injected to rats fasted for 24 h. Six hours after the administration the animals were euthanized. Reserpine was administered in a dose of 10 mg/kg intramuscular (i.m.) injected to 36 h fasted rats according to the method of Gupta (1974) [11]. The animals were euthanized 24 h after the reserpine administration. The stomachs were removed and the degree of ulceration was graded according to the following scale of severity [10]: 0 = no visible ulcer; 1 = petechial haemorrhage or pinpoint ulcers; 2 = one or two small ulcers; 3 = many ulcers, mainly small; 4 = many ulcers, mainly large.
Effects of AALE on cysteamine-induced duodenal lesions: Acute duodenal lesions were induced in rats using two oral doses of cysteamine HCI (300 mg/kg) at 4 h interval in 1 ml of distilled water. AALE (500 mg/kg, p.o.), was administered 1 h prior to cysteamine. A control group received only the vehicle [12]. The animals were euthanized at 48 h after the last administration of cysteamine. The duodenum was examined for lesions and scored as follows: 0, for protected duodenums; 1, for each lesion on mucosal surface; 2, for each marked ulcer or necrosis; 3, for perforation.
Effects of AALE on EtOH-induced gastroduodenal ulcers: Twenty four hours before the experiments, the rats were fasted and allowed access to water ad libitum. On the day of the experiment, animals were anaesthetized with ketamine/xylazine (40-90 mg/kg ket. i.p. + 5-15 mg/kg xil. i.p.) and the duodenum was ligated at a site 2 cm distal to the pylorus [13]. After ligation, rats were divided in 4 groups. The control group was received EtOH (1 ml, p.o.). Others groups were treated with AALE (125, 250 and 500 mg/kg, p.o.) followed by EtOH. One hour after the administration of EtOH, animals were euthanized. The stomachs and duodenums were removed to measure the lesion area, as described above.
Effects of AALE on the healing of EtOH-induced gastric ulcers: The method describes by Sibila et al. (2007) [14] was used to examine whether AALE was able to influence the healing of EtOH-induced gastric lesions, AALE (125 and 250 mg/kg, p.o.) was administered 1 h after EtOH (50%, 1 ml, rat). Control rats were treated with an equal volume of vehicle. Starting from 1 h after AALE administration, the rats received only water. One half of the rats of each treatment regimen were euthanized 3 h after EtOH; the other half, 24 h after EtOH exposure. The stomachs were removed and processed as described above.
Gastroprotective mechanism of action of AALE
Role of prostaglandins on AALE-induced gastroprotective effect in rat: To investigate the involvement of endogenous PG in the gastroprotection induced by AALE, indomethacin (10 mg/ kg, dissolved in NaHCO3 0.5%) was i.p. injected 60 min before AALE (500 mg/kg, p.o.) [15] followed by EtOH 1 h thereafter. The animals were euthanized 1 h after EtOH exposure and the gastric mucosal lesions were measured as described above.
Role of nitric oxide (NO) on AALE-induced gastroprotective effect in rat: Prior to treatment with AALE, animals were received a NO synthase inhibitor L-NNA (Nω-nitro-L-arginine, 40 mg/kg, i.p.) dissolved in saline 15 min before AALE (500 mg/kg, p.o.), followed by EtOH 1 h thereafter [16]. The animals were euthanized 1 h after EtOH exposure and the stomachs were processed as previously described above.
Role of non-protein sulfhydril groups on AALE-induced gastroprotective effect in rats: To investigate the involvement of endogenous non-protein-SH groups (NP-SH) in the gastroprotective effect of AALE, NEM (N-ethylmaleimide 10 mg/kg, dissolved in saline solution) was subcutaneously (s.c.) injected 30 min before the administration of AALE (500 mg/kg, p.o.), followed by EtOH 1 h thereafter [17]. The animals were euthanized 1 h after EtOH exposure and the stomachs were processed as previously described above.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com). Data were indicated as the mean ± standard error of the mean (SEM). The statistical significance of the difference between the lesion area of the treated group and that of the control group was calculated by using Student’s t-test.
Results
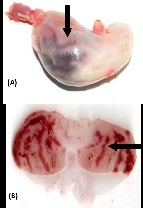
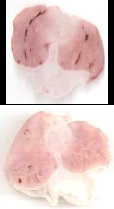
Effects of AALE on gastric ulcers induced by EtOH, HCI, NaOH and HCl/EtOH: The control group treated with EtOH presented an average ulcer area of 83.04 ± 8.00 mm2 , characterized by thick lines of black color (Figure 1A) and by hemorrhagic bands (Figure 1B). The reference drug carbenoxolone provided 95.10% gastroprotection (p< 0.001). Among the groups treated with AALE 250 or 500 mg/kg (Figure 2A and 2B) were exhibited a gastroprotective effect. AALE produced a highly significant inhibitory action against 0.6 N HCI, 0.2 N NaOH and 0.3 M/60% HCl/EtOH induced gastric lesions. The results are summarized in (Table 1).
Table 1: Effect of A. argentina (p.o.) on the gastric lesions induced by EtOH, HCI, NaOH and HCl/EtOH in rat.
Ulcer model and treatment |
Area of gastric lesion (mm2) |
Inhibition of damage (%) |
EtOH-induced ulcerogenesis |
|
|
EtOH absolute |
83.04 ± 8.00 |
- |
Carbenoxolone 250 mg/kg + EtOH absolute |
4.11 ± 1.21 *** |
95.10 |
AALE 250 mg/kg + EtOH absolute |
21.40 ± 2.96 *** |
74.23 |
AALE 500 mg/kg + EtOH absolute |
4.68 ± 1.52 *** |
94.37 |
HCl-induced ulcerogenesis |
|
|
HCI 0.6 N |
59.80 ± 8.98 |
- |
AALE 125 mg/kg + HCI 0.6 N |
11.25 ± 3.30 ** |
81.19 |
AALE 250 mg/kg + HCI 0.6 N |
10.75 ± 3.56 ** |
82.01 |
AALE 500 mg/kg + HCI 0.6 N |
1.25 ± 0.25 *** |
97.91 |
NaOH-induced ulcerogenesis |
|
|
NaOH 0.2 N |
68.75 ± 13.0 |
- |
AALE 125 mg/kg + NaOH 0.2 N |
0.34 ± 3.07 * |
70.42 |
AALE 250 mg/kg + NaOH 0.2 N |
49.33 ± 15.72 |
28.25 |
AALE 500 mg/kg + NaOH 0.2 N |
37.00 ± 9.07 |
46.19 |
HCl/EtOH-induced ulcerogenesis |
|
|
HCl/EtOH 0.3 M/60% |
51.68 ± 6.91 |
- |
AALE 250 mg/kg + HCl/EtOH 0.3 M/60% |
8.77 ± 3.22 ** |
83.04 |
AALE 500 mg/kg + HCl/EtOH 0.3 M/60% |
2.73 ± 1.58 ** |
94.72 |
Data are expressed as mean ± SEM. Six-eigth animals were used in each group. *p<0.05, **p<0.01, ***p<0.001 Student´s t-test as compared to respective controls. AALE (A. argentina lyophilized extract), EtOH (Ethanol), HCI (Hydrochloric Acid), NaOH (Sodium hydroxide) and HCl/EtOH (Hydrochloric Acid/ Ethanol).
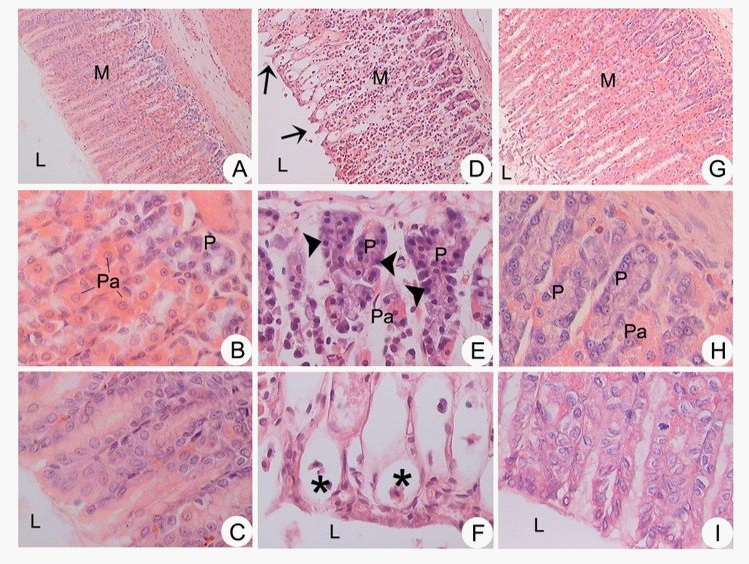
Histological examination of gastric ulcers induced by EtOH: Microscopic analysis revealed that AALE (500 mg/kg) maintained tissue integrity and prevented morphological changes in cells caused by EtOH. No histological changes were observed in stomach specimens taken from the normal group where the mucosa was intact (Figure 3 A-C). In contrast, ulcer control group exposure of rat to EtOH (Figure 3 D-F) produced regions with mucosal alteration compatible with zones of ulceration. The pretreatment with AALE improved the histological alterations caused by EtOH I). The gastroprotective effect of AALE was evidenced by decreasing hemorrhagic foci and ulceration zones.
Effects of AALE on gastric ulcers induced by acetylsalicylic acid, phenylbutazone and reserpine: Pretreatment with AALE produced a significant decrease in the intensity of ulceration induced by acetylsalicylic acid; however, the extract failed to protect animals against fenilbutazone and reserpine induced ulcers (Table 2).
Table 2: Effect of A. argentina (500 mg/kg, p.o.) on the gastric lesions induced by acetylsalicylic acid, phenylbutazone and reserpine in rat.
Ulcer model and treatment |
Scale of severity |
Acetylsalicylic acid-induced ulcerogenesis |
|
Acetylsalicylic acid 200 mg/kg |
3.58 ± 0.20 |
AALE 500 mg/kg + Acetylsalicylic acid 20 mg/kg |
1.16 ± 0.10 *** |
Phenylbutazone-induced ulcerogenesis |
|
Phenylbutazone 200 mg/kg |
2.16 ± 0.16 |
AALE 500 mg/kg + Phenylbutazone 200 mg/kg |
2.20 ± 0.37 |
Reserpine-induced ulcerogenesis |
|
Reserpine 10 mg/kg |
2.00 ± 0.31 |
AALE 500 + Reserpine 10 mg/kg |
1.00 ± 0.42 |
Data are expressed as mean ± S.E.M. Six-eigth animals were used in each group. ***p<0.001 Student´s t-test as compared to respective controls.
Data are expressed as mean ± S.E.M. Six-eigth animals were used in each group. ***p<0.001 Student´s t-test as compared to respective controls.
Effects on cysteamine-induced duodenal lesions: Cysteamine treatment produced duodenal ulcers in 90% of all the rats (1.25 ± 0.25). They were elongated, extending longitudinally down the duodenum and could easily be measured. AALE treatment reduced the average lesion score (0.90 ± 0.10), but this change was statistically not significant.
Effects of AALE on EtOH-induced gastroduodenal ulcers: In the stomach, edematous macroscopic areas were observed, with lesions that were visualized as hemorrhagic bands, which also manifested injury on the surface of the duodenum exposed to the action of EtOH. The pretreatment with AALE, in doses 125, 250 and 500 mg/kg, significantly reduced the gastroduodenal macroscopic injury with respect to group treated with EtOH (Table 3).
Table 3: Effects of A. argentina (125, 250 and 500 mg/kg, p.o.) on EtOH-induced gastroduodenal ulcers.
Treatment |
Area of gastric lesion (mm2) |
EtOH |
107.89 ± 24.03 |
AALE 125 mg/kg + EtOH |
6.81 ± 0.41 ** |
AALE 250 mg/kg + EtOH |
5.55 ± 2.20 ** |
AALE 500 mg/kg + EtOH |
4.18 ± 1.27 * |
Data are expressed as mean ± S.E.M. Six-eigth animals were used in each group. *p<0.05, **p<0.01 Student´s t-test as compared to respective control. AALE (A. argentina lyophilized extract), EtOH (Ethanol)
Effects of AALE on the healing of EtOH-induced gastric ulcers: As expected, 50% EtOH induced a gastric haemorragic damage. The evaluation of lesion area in AALE 125 and 250 mg/ kg treated rats throughout the experimental period reaching a reduction of 18.90% and 6.31%, respectively, of the initial score, 24 h after EtOH. The difference, however, reaches a statistical significance only 3 h in rats treated with AALE 250 mg/kg after EtOH (p<0.001) (Table 4).
Table 4: Effects of acute oral administration of A. argentina (125 and 250 mg/kg, p.o.) on the healing of gastric lesions induced by EtOH (50%, 1 ml, p.o.).
Treatment |
Area of gastric lesion (mm2) |
|
|
3 h |
24 h |
EtOH 50% |
35.33 ± 7.32 |
30.25 ± 5.00 |
EtOH 50% + AALE 125 mg/kg |
20.48 ± 7.44 |
5.72 ± 4.77 ** |
EtOH 50% + AALE 250 mg/kg |
3.41 ± 1.11 *** |
1.91 ± 1.11 ** |
The rats were given AALE 60 min after EtOH. Six-eigth animals were used in each group. Data are expressed as mean ± S.E.M. ***p<0.001, **p< 0.01. Student´s t-test as compared to respective controls. AALE (A. argentina lyophilized extract), EtOH (Ethanol).
Gastroprotective mechanism of action of AALE
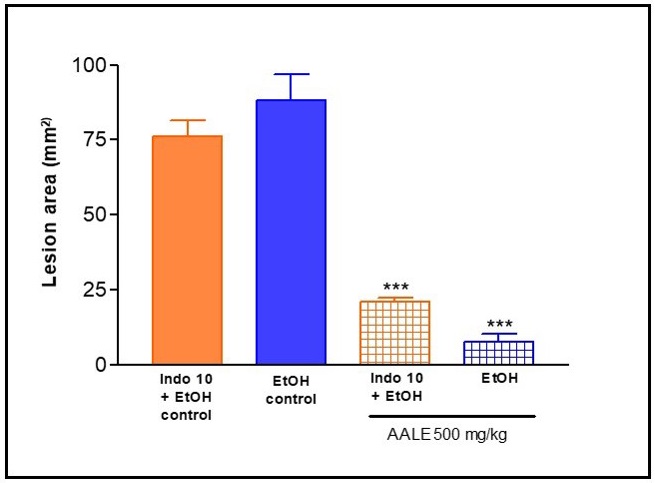
Role of prostaglandins on AALE-induced gastroprotective effect in rat: AALE (500 mg/kg) showed a significant decrease (p<0.001) in gastric injury induced by ethanol compared to vehicle control group. On the other hand indomethacin (10 mg/kg) pretreatment attenuated the protective effect of AALE. These results indicate that the gastroprotective effect of A. argentina is mediated, at least in part, by endogenous prostaglandins (Figure 4).
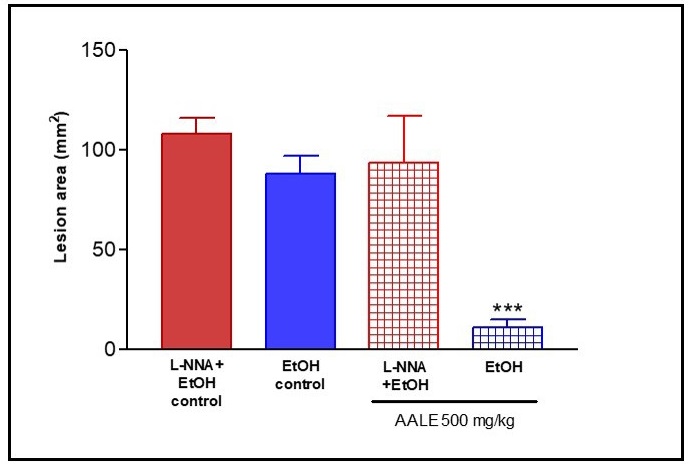
Role of nitric oxide (NO) on AALE-induced gastroprotective effect in rat: Pretreatment of L -NNA, an inhibitor of nitric oxide synthase, increased EtOH-induced gastric mucosal lesions. L -NNA attenuating significantly the protective effects of AALE (500 mg/kg) (Figure 5). On the basis of this evidence it can be suggested that gastroprotective effect of A. argentina might be, in part, through nitric oxide.
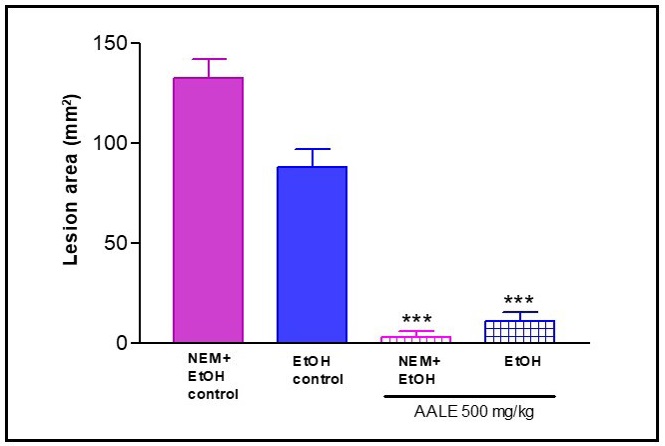
Role of non-protein sulfhydril groups on AALE-induced gastroprotective effect in rats: Pretreatment with NEM, a SHblocker, did not alter the protection induced by AALE. These results suggest that the gastroprotective effect of A. argentina no involves non-protein sulfhydril groups (Figure 6).
Discussion
In this study, it was evaluated the gastroprotective effect of AALE extract in different models of induced-gastric ulcers (ethanol, NSAIDs, reserpine, cysteamine) and model of ethanol-induced gastroduodenal ulcers, as well as, effect on the healing of ethanol-induced gastric ulcers. Also were investigations of the possible mechanisms of action involved in the gastroprotective activity of extract. Gastric ulcer and lesions, that usually occur in duodenum, are considered the most wide diseases the world and, as well as affecting millions of people [18]. Gastric ulcer, a chronic multifactorial disease resulting from an imbalance between the protective and aggressive factors of the gastrointestinal mucosa. The healing process plays a critical role in the treatment of gastric ulcer.
Gastric mucosa is frequently expose to food products, providing vital nutrients to the organism. However, some foods and drugs delivered via the oral route can disturb gastric mucosal integrity. Factors major of causative the gastric ulcers are ethanol, nicotine and ingestion of drugs, as nonsteroidal antiinflammatory drugs (NSAIDs) (e.g., acetylsalicylic acid) [15]. Among the risk factors of peptic ulcer disease were described H. pylori infection, the exposure to chronic stress, and ischemia to the gastric tissue. Ethanol-induced gastric ulcer model is a widely accepted in the evaluation of vegetable extracts to target gastroprotective activity due to its good reproducibility because ethanol is one of factors that increase the risk of ulcer formation. Ethanol-induced gastric mucosa lesions can be produced reliably and simply by intragastric administration of varying amounts (0.5-2.0 ml) of concentrated (50- 100%) ethanol. The severity of the injury is relate with the doses of ethanol. Absolute ethanol induces severe histopathological changes in mucosa of mouse and rat stomach consisting of acute erosive hemorrhagic lesions and ulcers when they are examined 1-2 h after ethanol administration [19].
After of administration ethanol, it produces depletion in the pre-epithelial defense components and a destabilization of blood flow. It penetrates the gastric mucosa due to its ability to solubilize the protective mucous and expose the mucosa to actions of hydrochloric acid and pepsin, inducing damage to membrane [20]. Moreover, ethanol stimulates acid secretion and reduces blood flow leading to microvascular injuries, through disruption of the vascular endothelium and facilitating vascular permeability; it also increment activity of xanthine oxidase and it is responsible imbalances in cellular antioxidant processes. Ethanol induced membrane injury is associated with increased permeability of the plasma membrane by sodium and water. It also produces massive intracellular increase of calcium, it a major step in the pathogenesis of gastric mucosa injury. This promove to cell death and exfoliation in the surface and ethanol-induced ulceration is linked to increment apoptosis [21].
The mucosa ability to resist gastric injury requires the integrity of cytoprotective factors such as the pre-epithelial mucus bicarbonate layer, the intercellular tight junctions connecting the epithelial cells, the presence of prostaglandins, cytokines, enteric nerves and blood flow [22]. The development of gastric lesions by necrotizing agents implicate the reduction of protective mechanisms. The association of HCl/EtOH produce gastric lesions by promoting stasis in gastric blood flow that conduce to the development of the hemorrhagic and necrotic aspects. AALE showed significant effect in decrease lesion area gastric mucosa triggered by necrotizing agents as such ethanol, HCl and NaOH; these agents were known as to promote oxygen free radicals [23].
Oxidative stress plays an important role in the pathogenesis of various diseases including gastric ulcer. It has reported that antioxidants play a significant role in the protection of gastric mucosa against various necrotic agents [1]. Borneo et al. (2009) [24] studied the antioxidant activity the extracts of 41 Cordo-ba’ plants, including A. argentina, using the Ferric Reducing Antioxidant Power (FRAP) assay, which is, based on the ability to reduce ferric (III) iron to ferrous (II) iron. The medicinal plants were grouped into four categories: low antioxidant power (<100 μmol Fe (II)/g), medium (100–250 μmol Fe (II)/g), high (250–625 μmol Fe (II)/g), extremely high (>625 μmol Fe (II)/g). A. argentina showed a high antioxidant activity of 265.8 μmol of Fe (II)/g. Therefore, it could be conceivable that the anti-ulcerogenic activity of AALE could be linked to the antioxidant capacity.
On the other hand, the results on histological analysis on gastric mucosa of rat treated with ethanol, results in acute, hemorrhagic, band-like necrosis of the mucosa confined to superficial of thickness, but in the group pre-treatment with AALE extract, the mucosa appears to be more preserved. The ethanol-induced congestion, haemorrhage, edema, necrosis, inflammatory and dysplastic changes, erosions and ulceration were inhibited by AALE. The cytoprotective effects of AALE was tested on gastric ulcers induced by acetylsalicylic acid, phenylbutazone, reserpine and cysteamine. Acetylsalicylic acid is a well known gastric mucosal barrier breaker [25] and phenylbutazone is known to inhibit mucopolysaccaride synthesis by gastric mucosal cells, or to reduce their mitotic activity, or both [26]. Ulcerogenic activity of reserpine has been attributed to the degranulation of gastric mast cells due to liberation of histamine, presume to be mediated by the cholinergic system [27]. Our results indicated that AALE provided significant gastric protection in lesions induced with acetylsalicylic acid.
Other model of peptic ulcer disease is treatment with cysteamine. It stimulates gastric acid secretion rate and inhibits the alkaline mucus secretion from Brunner’s glands in the proximal duodenum resulting in the formation of duodenal ulcer [28]. Our study showed a decrease in the duodenal ulcers induced by cysteamine, but was not statistically significant after oral pretreatment with AALE. Another line of the study was to evaluate the healing of ethanol induced gastric lesions. The ulcer healing process is complicated and modulated by multiple pro-inflammatory cytokines, growth factors and proteases. That platelets play an important role in ulcer healing over and above their role in coagulation by releasing many growth factors in the sites of injury that enhance epithelial and endothelial cell proliferation required for gastric mucosal repair. Gastric ulcer healing, in fact, can be accelerated by the release of angiogenic stimulators such as VEGF, PDEGF and EGF stored in platelets or inhibited by the increased release of endostatin, an anti-angiogenic factor [29]. AALE significantly accelered healing on gastric lesions ethanol-induced after 3 h. However, further studies are required to confirm the involvement of such growth factors in the behaviour of extract on gastric ulcer healing.
On the other hand, AALE administered before ethanol treatment greatly reduced the macroscopic gastroduodenal damage. There are evidences indicated that generation of oxygen free radicals following ethanol administration may be causative in these lesions. NSAIDs such as indomethacin, acetylsalicylic acid and ibuprofen are known to cause gastric ulcers, especially when abused. This phenomenon has been employed in the development of NSAIDs-induced gastric ulcer models in rats by suppression of prostaglandin synthesis in the cyclooxygenase (COX) pathway. The two isoforms of COX (COX-1 and COX-2), acts on arachidonic acid to form prostaglandins E2 (PGE2) and prostacyclin (PGI2). PGE2 and PGI2 are potent vasodilators and are involved in the maintenance of homeostasis and gastric ulcer healing. Role of prostaglandins is to stimulate the secretion of bicarbonate and mucus, maintaining gastric mucosa blood flow and regulating mucosal cell turnover and repair. Conventional NSAIDs cause gastric damage with a decrease in mucosal PGE2 production, irrespective of the route of administration [30].
The pretreatment with indomethacin on ethanol-induced gastric lesions attenuated the gastroprotective effect of AALE, compared to the effect of extract in the absence of such blocking, suggesting that prostaglandins are partially involved in this activity. Another class of substances that play a cytoprotective role in gastric mucosa is NO. Physiologically, NO is produced from L-arginine by constitutive NO synthase (NOS) and inducible NOS enzymatic pathways. NO is involved in the regulation of gastric blood flow (GBF), decreased neutrophil infiltration, stimulation of angiogenesis and the maintenance of gastric mucosal barrier integrity in either healthy or that damaged by strong irritant. This is supported by the observation that the inhibition of NO production by L-NNA not only markedly impaired gastric secretion and gastric motility but also abolished the protective activity of gastroprotective agents [31]. This study showed the pretreatment with L-NNA on ethanol-induced gastric lesions decrease the gastroprotective effect of AALE. That blockade of NO synthesis greatly increased the susceptibility of the stomach to damage induced by ethanol. Evidence was suggesting that NO is involved at least in part in the gastroprotective effect of the extract.
Non-protein sulphydryl compounds are important components of the gastric mucus. The NP-SH exert functions in gastroprotection by maintaining the integrity of the mucosal barrier and binding free radicals formed due to the action of noxious agents like the ethanol [32]. The raise in mucosa damage by noxious agents is generally accompanied by a decrease in NPSH compounds amount. In this way, we investigated the possible involvement of endogenous NP-SHs in the gastroprotective effect of the AALE. Pretreatment with NEM did not alter the protection induced by AALE. On the other hand, antidiarrheal activity is an advantage for the antiulcerogenic agents because relaxation of circular muscles and flattening of the folds in the stomach lead to an increase in the mucosal area exposed to the necrotizing agent, decreasing the incidence of ulcers [33]. Treatment of diarrhea can be performed through decreasing peristalsis or decreasing secretion from the intestinal mucosa. A. argentina offered antidiarrheal activity through decreasing the incidence of watery stools, possibly through inhibition the gastrointestinal motility and inhibition the intraluminal fluid. The α2 -adrenoreceptor antagonists (yohimbine and phentolamine) counteracted the effects of AALE on intestinal transit. This indicates a role for the α2 -adrenergic system in the action antidiarrheal activity of AALE. The effect of AALE on intestinal motility was affected by naloxone, delays small intestinal transit possibly, at least in part, involving opioid receptors, which are found in the gastrointestinal wall [6]. In relation to everything mentioned above, there are data suggested that presynaptic α2 -adrenoceptors and opioid peptides can mediate gastric mucosa protection [34]. AALE could exert its cytoprotective effect through these routes.
On the other hand, it is known in over half of the world's population H. pylori colonizes the stomach and is intimately associated with the gastric disorders ranging from chronic gastritis and peptic ulcers to gastric cancer. After entering the host stomach, H. pylori use its urease activity to neutralize the hostile acidic condition at the starting of infection. This promove the inflammation process and pathologic changes in gastric mucosa. The bacterium converts urea into ammonia and carbon dioxide modifying the acidic gastric environment to facilitate colonization. Others studies indicate that H. pylori can exist both in human gastric mucosa and on abiotic surface forming biofilms, explaining the ability of the organism to survive within and outside the host. It has been reported that A. argentina was very effective against H. pylori strains sensible and resistant and in planktonic or biofilm state. Besides, it has been proven to be an urease inhibitor, which might contribute to counteract the colonizing and virulent factor of H. pylori biofilm [35]. Plant extracts can be behave as a new alternative for the treatment of gastric disorders because the biochemical constituents can perform as a phytocomplex persuading the lessening of the effects of the offensive agents and strengthening of the defensive mechanisms.
Allantoine identified in A. argentina [36], may be responsible, at least in part, of the cytoprotective activity. Moreira da Silva et al. (2018) [37] provided evidence of the gastroprotective activity of allantoin against common necrotizing agents, such as stress, NSAID, and ethanol; finding that the antisecretory and cytoprotective mechanisms are probably associated with an increase in PGE2 levels.
Conclusion
In conclusion, this study showed gastroprotective activity of the aqueous extract obtained from the roots of A. argentina in different models in rats. A. argentina inhibited gastric lesions induced by ulcerogenic agents and possibly involving PG and NO in attenuating the gastroprotective process. All secondary metabolites play their substantial role supporting the pharmacological activity and effectiveness of A. argentina.
Declarations
Conflicts of Interest: All authors declare no conflict of interest.
Acknowledgements: The authors are grateful to Dr. María Fusco and Dr. Ángela Sosa, Farmacognosy, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, Argentina, for providing infusion of A. argentina and to staff of the Bioterio, Mr. Manuel Arroyuelo and Mrs Silvina García.
Funding: This work has been supported by the research project: PROICO 2-4218, of the Secretaría de Ciencia y Tecnología of Universidad Nacional de San Luis, Argentina.
References
- Da Silva Junior IF, Balogun SO, Oliveira RG, Damazo AS, Oliveira Martins DT. Piper umbellatum L.: A medicinal plant with gastriculcer protective and ulcer healing effects in experimental rodent models. J Ethnopharmacol. 2016; 192: 123-131.
- Arroyo J, Bonilla P, Moreno-Exebio L, Ronceros G, Tomás G, Huamán J, et al. Efecto gastroprotector y antisecretor de un fitofármaco de hojas de matico (Piper aduncum). Rev Peru Med Exp Salud Publica. 2013; 30: 608-615.
- Zhang Y, Sun H, Chen X, Li J, Zhao H, Geng L, et al. Functional profile of gastric epithelial cells infected with Helicobacter pylori strains. Microb Pathog. 2016; 95: 77-81.
- Barboza GE, Cantero JJ, Núñez C, Pacciaroni A, Ariza Espinar L. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana. 2009; 34: 7-365.
- Priestap H, Van Baren C, Di Leo Lira P, Coussio J, Bandoni A. Volatile constituents of Aristolochia argentina. Phytochemistry. 2003; 63: 221-225.
- Paredes JD, Sosa A, Fusco M, Teves MR, Wendel GH, Pelzer LE. Antidiarrhoeal activity of Aristolochia argentina Gris. (Aristolochiaceae) in rodents. J Appl Pharm Sci. 2016; 6: 146-152.
- Robert A, Nezamis JE, Lancaster C. Cytoprotection by prostaglandins in rat prevention of gastric necrosis produced by alcohol, HCI, NaOH, hypertonic-NaCl and thermal injury. Gastroenterology. 1979; 77: 433-443.
- Khan HA. Computed assisted visualization and quantitation of experimental gastric lesions in rats. J Pharmacol Toxicol Methods. 2004; 49: 89-95.
- Hemmati H, Revani A, Djhaguiri B. Prevention of aspirin-induced ulceration in rats with methyldopa and disulfiram. Pharmacology.1973; 9: 374-378.
- Galil A, Marshall PB. Phenylbutazone and histamine in rat glandular stomach: its relationship to gastric ulceration. Br J Pharmacol Chemother. 1968; 33: 1-14.
- Gupta MB, Tangri KK, Bhargava KP. Mechanism of ulcerogenic activity of reserpine in albino rats. Eur J Pharmacol. 1974; 27: 269-272.
- Selye H, Szabo S. Experimental model for production of perforating duodenal ulcer by cysteamine in rat. Nature. 1973; 244: 458-459.
- Melchiorri D, Sewerynek E, Reiter RJ, Ortiz GG, Poeggeler B, Nistico G. Suppressive effect of melatonin administration on etanol-induced gastroduodenal injury in rats in vivo. Br J Pharmacol. 1997; 121: 264-270.
- Sibila V, Pagani F, Lattuada N, De Luca V, Guidobono F, Soglian A, et al. Ticlopidine prevents the formation but delays the healing of ethanol-induced gastric lesions in the rat. Pharm Res. 2007; 55: 418-425.
- Chávez-Piña AE, Tapia-Álvarez GR, Navarrete A. Inhibition of endogenous hydrogen sulfide synthesis by PAG protects against ethanol-induced gastric damage in the rat. Eur J Pharmacol. 2012; 630: 131-136.
- Jiménez D, Martin MJ, Pozo D, Alarcón C, Esteban J, Bruseghini L, et al. Mechanisms involved in protection afforded by L-arginine in ibuprofen-induced gastric damage: role of nitric oxide and prostaglandins. Dig Dis Sci. 2002; 47: 44-53.
- Matsuda H, Li Y, Yoshikawa M. Gastroprotections of escins Ia, Ib, IIa and IIb on etanol-induced gastric mucosal lesions in rats. Eur J Pharmacol. 1999; 373: 63-70.
- Sousa Glaubert A, Oliveira Irisdalva S, Silva-Freitas FV, Viana AFSC, Neto BPS, Cunha FVM, et al. Gastroprotective effect of ethanol extracts of cladodes and roots of Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. Ex Rowl (Cactaceae) on experimental ulcer models. J Ethnopharmacol. 2018; 218: 100-108.
- Szabo S. Mechanisms of mucosal injury in the stomach and duodenum: time-sequence analysis of morphologic, functional, biochemical and histochemical studies. Scand J Gastroenterol. 1987; 22: 21-28.
- Sener G, Paskaloglu K, Ayanoglu-Dulger G. Protective effect of increasing doses of famotidine, omeprazole, lansoprazole, and melatonin against ethanol-induced gastric damage in rats. Indian J Pharmacol. 2004; 36: 171-174.
- Hernández Muñoz R, Montiel Ruiz C, Vazquez Martinez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Investig. 2000; 80: 1161-1169.
- Ham M, Kaunitz JD. Gastroduodenal defense. Curr Opin Gastroenterol. 2007; 23: 607-616.
- Szelenyi I, Brune K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. 1998; 33: 865-871.
- Borneo R, León AE, Aguirre A, Ribotta P, Cantero JJ. Antioxidant capacity of medicinal plants from the Province of Córdoba (Argentina) and their in vitro testing in a model food system. Food Chem. 2009; 112: 664-670.
- Devenport HW. Salicylate damage to the gastric mucosal barrier. N Engl J Med. 1976; 276: 1307-1312.
- Bucciarelli G, Biliotti G, Andreoli F, Marshi C, Michel E. The action of phenylbutazone on mitotic activity of gastric antrum cells. Sperimentale.1968; 118: 469-474.
- Singh S. Evaluation of gastric anti-ulcer activity of fixed oil of Ocimum basilicum Linn. and its possible mechanism of action. Indian J Exp Biol.1999; 37: 253-257.
- Poulsen SS, Olsen SP, Kirkegaard P. Healing of cysteamine-induced duodenal ulcers in the rat. Dig Dis Sci. 1985; 30: 161-167.
- Perini R, Wallace JL, Ma L. Roles of platelets and proteinase-activated receptors in gastric ulcer healing. Dig Dis Sci. 2005; 50: S12-S5.
- Takeuchi K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol. 2012; 18: 2147-2160.
- Kuo P, Gentilcore D, Nair N, Stevens JE, Wishart JM, Lange K, et al. The nitric oxide synthase inhibitor, Ng-nitro-L-argininemethyl ester, attenuates the delay in gastric emptying induced by hyperglycemia in healthy humans. Neurogastroenterol Motil. 2009; 21: 1175-e103.
- Szabo S, Vattay P. Experimental gastric and duodenal ulcers. Advances in pathogenesis. Gastroenterol Clin N Am. 1990; 19: 67- 85.
- Wasman SQ, Mahmood AA, Salehhuddin H, Zahra AA, Salmah I. Cytoprotective activities of Polygonum minus aqueous leaf extract on ethanol-induced gastric ulcer in rats. J Med Plant Res. 2010; 4: 2658-2665.
- Gyires K, Zadori ZS, Shujaa N, Al-Khrasani M, Pap B, Mózes MM, et al. Pharmacological analysis of α2-adrenoceptor subtypes mediating analgesic, antiinflammatory and gastroprotective actions. Inflammopharmacology. 2009; 17: 171-179.
- Salinas Ibáñez AG, Arismendi Sosa AC, Ferramola FF, Paredes J, Wendel G, Maria AO, et al. Inhibition of Helicobacter pylori and Its Associated Urease by Two Regional Plants of San Luis Argentina. Int J Cur Microbiol Appl Sci. 2017; 6: 2097-2106.
- Priestap HA, Ruveda EA, Albonico SM. The alkaloids from the roots of Aristolochia argentina Gris. Anales Asoc Quím Argentina. 1972; 60: 308-316.
- Moreira da Silva D, Rodrigues Martins JL, Ramos de Oliveira D, Ferreira Florentino I, Bueno da Silva DP, Alcântara dos Santos FC, et al. Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur J Pharmacol. 2018; 821: 6878.