Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 1
Histopathologic spectrum of colonoscopic findings from patients with positive multitarget stool DNA test (Cologuard™): A single institutional experience
Kuixing Zhang1; Ari Kassardjian2; Changli Lu3; Hanlin L Wang4*
1 Yosemite Pathology Medical Group, Modesto, CA, USA.
2 Kaiser Permanente Woodland Hills Medical Center, Los Angeles, CA, USA.
3 Department of Pathology, Huaxi Hospital of Sichuan University, Chengdu, Sichuan, China.
4 Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
*Corresponding Author : Hanlin L Wang
Department of Pathology and Laboratory Medicine,
David Geffen School of Medicine at UCLA, 10833 Le
Conte Avenue, Los Angeles, CA 90095, United States.
Email: hanlinwang@mednet.ucla.edu
Received : Oct 25, 2021
Accepted : Dec 08, 2021
Published : Dec 13, 2021
Archived : www.jjgastro.com
Copyright : © Wang HL (2021).
Abstract
Background: In 2014, the FDA approved the first stool DNA-based multitarget colorectal cancer (CRC) screening test, the CologuardTM test. It has shown a higher sensitivity but a lower specificity than the other stool-based non-invasive test, the fecal immunochemical test, for the detection of CRC and advanced precancerous lesions. There have been limited studies on the positive predictive value (PPV) of the CologuardTM test.
Methods: A retrospective clinicopathologic review was performed on 147 patients who were tested positive for CologuardTM and followed by diagnostic colonoscopy with biopsies/polypectomies in our institution from 2016 to 2021.
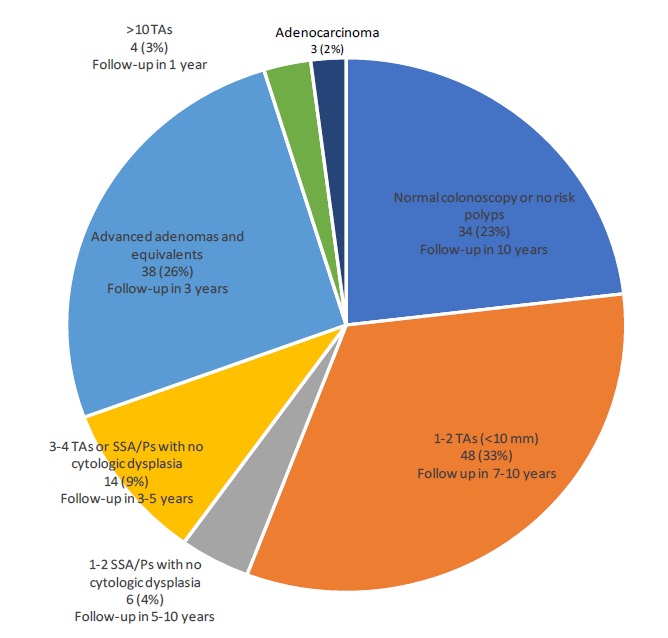
Results: On colonoscopy and subsequent histopathologic examination, 3 patients (2.0%) were found to have invasive adenocarcinoma. There were 110 patients (74.8%) who had any precancerous lesion such as tubular adenoma or sessile serrated adenoma/polyp (SSA/P). Among them, 42 (28.6%) had high-risk precancerous lesions such as advanced adenoma, traditional serrated adenoma, SSA/P with cytologic dysplasia, or ≥5 adenomas. There were 34 patients (23.1%) who showed no carcinoma or precancerous lesion during the subsequent colonoscopy. Among them, 15 (10.2%) had hyperplastic polyps, which were all <10 mm in size and <20 in number.
Conclusion: CologuardTM test offers a convenient, non-invasive option for CRC screening. It shows a high PPV for precancerous lesions but a low PPV for CRC. Thus, a positive CologuardTM test result should always be followed by diagnostic colonoscopy, which remains to be the gold standard for CRC detection in the era of molecular medicine.
Keywords: colorectal cancer; adenocarcinoma; cologuard; multitarget stool DNA test; adenoma; sessile serrated adenoma/polyp; screening colonoscopy; positive predictive value.
Abbreviations: CRC: colorectal cancer; PPV: positive predictive value; SSA/P: sessile serrated adenoma/ polyp; US: United States; ACS: American Cancer Society; ACG: American College of Gastroenterology; CT: computed tomography; FIT: fecal immunochemical test; gFOBT: guaiac-based fecal occult blood test; mt-sDNA: multitarget stool DNA; TSA: traditional serrated adenoma; FDA: US Food and Drug Administration.
Citation: Zhang K, Kassardjian A, Lu C, Wang HL. Histopathologic spectrum of colonoscopic findings from patients with positive multitarget stool DNA test (Cologuard™): A single institutional experience. Japanese J Gastroenterol Res. 2021; 1(9): 1044.
Introduction
In the United States (US), colorectal cancer (CRC) is the fourth most commonly diagnosed and the second deadliest cancer, with estimated 147,950 new cases and 53,200 deaths in 2020 [1]. If detected early, up to 90% of deaths can be prevented [2]. An effective strategy to reduce CRC incidence and to improve overall survival is regular CRC screening. In the US, the CRC screening program guidelines rely on both government institutions and national independent bodies, such as the US Preventive Services Task Force, the American Cancer Society (ACS) and the American College of Gastroenterology (ACG). The most current ACG guidelines published in 2021 recommended CRC screening for average-risk individuals aged 50 to 75 years [3]. Screening for average-risk individuals aged 45 to 49 years was also suggested. For individuals beyond age 75 years, the ACG guidelines suggested that the decision to continue CRC screening be individualized. The ACS guidelines published in 2018 recommended that screening begin at the age of 45 and discontinue at the age of 85 [4]. In terms of options for CRC screening, the ACS guidelines included visual invasive examinations, such as computed tomography (CT) colonography (every 5 years), flexible sigmoidoscopy (every 5 years) or colonoscopy (every 10 years) [4]. The ACS guidelines also recommended stool-based non-invasive tests, such as fecal immunochemical test (FIT) every year. In fact, FIT has been gradually replacing the guaiac-based fecal occult blood test (gFOBT) and has become the most commonly used screening tool for CRC globally. Along with colonoscopy, it is also the primary screening modality recommended by the ACG [3]. FIT enables detection of a small amount of blood by targeting hemoglobin using antibodies that selectively detect the globin portion of human hemoglobin. gFOBT is no longer recommended due to its high false-positive rate as well as the dietary and pharmaceutical restrictions [5,6]. Despite the availability of multiple screening tools to improve early detection of CRC, nearly one-third of eligible US adults have never been screened, well below the National Colorectal Cancer Roundtable goal of 80% adherence among eligible adults [7]. While improvements in FIT detection is still ongoing, the need for more accurate non-invasive tests remains.
Since enterocytes constantly exfoliate and shed into the intestinal lumen, molecular alterations in feces, such as DNA methylations, have been widely investigated [8]. The multitarget stool DNA (mt-sDNA) test (also called Cologuard™) is a non-invasive CRC screening tool, developed by Exact Sciences Corporation and Mayo Clinic [9]. Cologuard™ is designed to detect three independent categories of biomarkers that exhibit an additive association with CRC. The first category targets epigenetic changes in the form of gene promoter methylation. The specific methylated gene targets include N-Myc DownstreamRegulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3). The second targets seven specific mutations in the v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) gene. The third biomarker is non-DNA-based and detects occult hemoglobin. Additionally, β-actin is used as a reference gene for confirmation and quantitative estimation of the total amount of human DNA present in each sample. Results from the methylation, mutation and hemoglobin assays are integrated by the Exact Sciences Analysis Software to determine a positive or negative reportable result or an invalid result [10].
Cologuard™ is so far the only fecal-based mt-sDNA test marketed in the US. In a cross-sectional study at 90 sites throughout the US and Canada involving 9,989 participants, 65 (0.7%) patients were found to have CRC, 757 (7.6%) had advanced precancerous lesions (advanced adenomas or sessile serrated polyps measuring ≥1 cm), 2,893 (29%) had non-advanced adenomas, and 6,274 (62.8%) had negative findings on colonoscopy. The sensitivity of mt-sDNA test for CRC detection was 92.3%, compared with 73.8% for FIT. For participants with a negative colonoscopy, the specificity of mt-sDNA test was significantly lower than that for FIT: 89.8% versus 96.4%, respectively, indicating a higher false-positive rate with mt-sDNA test [11], which led to unnecessary colonoscopy in some patients.
This study investigated the spectrum of colonoscopic and histopathologic findings in patients with a positive Cologuard™ test in our institution. The goals were to determine the false positive rate and the positive predictive rate for CRC as well as its precursor lesions in our patient cohort.
Materials and methods
Patient population and data source
This study was approved by the University of California Los Angeles (UCLA) Institutional Review Board. The database of patients who tested positive with the Cologuard™ test and subsequently underwent colonoscopy with biopsies/polypectomies at UCLA from 2016 to 2021 were retrospectively reviewed.
All colonoscopies were performed by experienced gastroenterologists according to standard protocol. Colonoscopically detected polyps were described according to their location, size, and microscopic diagnosis. The lesions detected by colonoscopy were stratified into various risk groups according to the surveillance colonoscopy interval recommended by the US Multi-Society Task Force on Colorectal Cancer [12]: (1) adenocarcinoma; (2) >10 adenomas on single examination (recommended surveillance colonoscopy interval of 1 year); (3) advanced adenoma (adenoma with villous or tubulovillous histology, with highgrade dysplasia, or ≥10 mm in size), 5-10 adenomas <10 mm, traditional serrated adenoma (TSA), 5-10 sessile serrated adenomas/polyps (SSA/P) <10 mm, SSA/P ≥10 mm, or SSA/P with cytologic dysplasia (recommended interval of 3 years); (4) 3-4 tubular adenomas <10 mm, 3-4 SSA/Ps, or hyperplastic polyp ≥10 mm (recommended interval of 3-5 years); (5) 1-2 SSA/Ps <10 mm (recommended interval of 5-10 years); (6) 1-2 tubular adenomas <10 mm (recommended interval of 7-10 years); and (7) normal colonoscopy, ≤20 hyperplastic polyps <10 mm, or other no risk polyps (recommended interval of 10 years).
Statistical analysis
Patient and polyp characteristics were presented as a mean and range for continuous variables, and frequency and percentage for categorical variables. Microsoft Office-365 Excel (version 2016) was used for tabulation of the data and analysis.
Results
We retrospectively analyzed the data of 147 patients who had a positive Cologuard™ test and subsequently underwent colonoscopy with biopsies/polypectomies. There were 64 males (43.5%) and 83 females (56.5%) in this study. The patients’ age ranged from 33 to 87 years, with a mean age of 69.5 ± 9.4 and a median age of 71. The number of polyps detected in these patients ranged from 0 to 15, with a mean number of 2.9.
As shown in (Table 1) and (Figure 1), 3 patients (2.0%) were found to have invasive colorectal adenocarcinoma. These patients ranged in age from 64 to 75 years old. Two had stage III disease and one had stage I disease at the time of surgical resection. None of the patients had distant metastasis.
Table 1: Spectrum of colonoscopic and histopathologic findings (n=147).
Category |
No. (%) |
Normal colonoscopy or no risk polyps |
34 (23.1) |
≤20 hyperplasic polyp (<10 mm) |
15 (10.2) |
1-2 tubular adenoma(s) (<10 mm) |
48 (32.6) |
3-4 tubular adenomas (<10 mm) |
7 (4.8) |
5-10 tubular adenomas (<10 mm) |
2 (1.4) |
>10 tubular adenomas |
4 (2.7) |
Tubular adenoma (≥10 mm)* |
7 (4.8) |
Tubulovillous or villous adenoma* |
20 (13.6) |
Traditional serrated adenoma |
2 (1.4) |
Adenoma with high-grade dysplasia* |
2 (1.4) |
1-2 SSA/P with no cytologic dysplasia (<10 mm) |
6 (4.1) |
3-4 SSA/P with no cytologic dysplasia (<10 mm) |
1 (0.7) |
3-4 tubular adenoma and SSA/P with no cytologic dysplasia (<10 mm) |
6 (4.1) |
5-10 SSA/P with no cytologic dysplasia (<10 mm) |
1 (0.7) |
>10 SSA/P |
0 |
SSA/P ≥10 mm |
2 (1.4) |
SSA/P with cytologic dysplasia |
2 (1.4) |
Hyperplastic polyp (≥10 mm) |
0 |
Serrated polyposis syndrome |
0 |
Adenocarcinoma |
3 (2.0) |
SSA/P, sessile serrate adenoma/polyp *Adenomas with villous or tubulovillous histology, with high-grade dysplasia, or ≥10 mm in size are defined as advanced adenomas. These 3 features frequently overlap. To avoid double counting, adenomas with 2 or 3 advanced features were counted under the “villous or tubulovillous” category first. The remaining advanced adenomas (without villous or tubulovillous histology) were then counted under the size category (i.e., ≥10 mm). The rest of advanced adenomas (i.e., without villous or tubulovillous histology and <10 mm) were counted under the “high-grade dysplasia” category.
There were 34 patients (23.1%) who had a positive Cologuard™ test but with no carcinoma or precancerous lesions detected in the subsequent colonoscopy. Among them, 15 patients (10.2%) had hyperplastic polyps, which were all <10 mm in size and <20 in number. None of the patients met the criteria of serrated polyposis syndrome. Six patients (4.1%) had completely normal colonoscopic examination. Two patients (1.4%) had an inflammatory polyp. The remaining 11 patients had small colonic polyps that showed either benign lymphoid aggregates, mucosal prolapse or mucosal fold on histologic examination. The risk-stratified repeat colonoscopy interval for these patients would be 10 years according to the current recommendations by the US Multi-Society Task Force on Colorectal Cancer [12].
Among the patients with precancerous polyps detected by colonoscopy, 48 (32.6%) had only 1-2 small (<10 mm) tubular adenomas with no high-grade dysplasia. The risk-stratified repeat colonoscopy interval for these patients would be 7-10 years. There were also 6 patients (4.1%) who had only 1-2 small (<10 mm) SSA/Ps without cytologic dysplasia. The risk-stratified repeat colonoscopy interval for these patients would be 5-10 years.
There were 14 patients (9.5%) who had 3-4 small (<10 mm) tubular adenomas, 3-4 small (<10 mm) SSA/Ps, or 3-4 small (<10 mm) tubular adenomas and SSA/Ps without cytologic dysplasia. The risk-stratified repeat colonoscopy interval for these patients would be 3-5 years.
Twenty-nine patients (19.7%) had advanced adenomas defined by having villous or tubulovillous histology, having highgrade dysplasia, or being ≥10 mm in size. Additional 9 patients had TSA (n=2), SSA/P with cytologic dysplasia (n=2), large (≥10 mm) SSA/P (n=2), 5-10 small (<10 mm) tubular adenomas (n=2), or 5-10 small (<10 mm) SSA/Ps without cytologic dysplasia (n=1). The risk-stratified repeat colonoscopy interval for these patients would be 3 years. There were only 4 patients (2.7%) who had >10 tubular or tubulovillous adenomas on one single examination. The risk-stratified repeat colonoscopy interval for these patients would be 1 year.
Overall, in our cohort, the positive predictive value (PPV) of the Cologuard™ test was 2.0% for colonic adenocarcinoma; 30.6% for adenocarcinoma and high-risk precancerous lesions such as advanced adenoma, TSA, SSA/P with cytologic dysplasia or ≥5 adenomas (28.6% for high-risk precancerous lesions only); and 76.9% for adenocarcinoma and any precancerous lesion (74.8% for any precancerous lesion only). The false positive rate of the Cologuard™ test in our patient population was 23.1%.
Discussion
The mt-sDNA test, sold in the US under the brand name of Cologuard™, was approved in 2014 by the US Food and Drug Administration (FDA) and the US Preventive Services Task Force as an accepted CRC screening option [9]. The Centers for Medicare and Medicaid Services have included coverage for this test for individuals between the ages of 50 and 84 years who are considered average-risk patients. The performance of the mt-sDNA test for CRC screening has been evaluated in several large-scale studies worldwide [11,13-15]. Overall, the mt-sDNA test is more sensitive but less specific than FIT in detecting CRC and precancerous lesions. These data make sense given the fact that FIT detects only one of the three categories of the mt-sDNA test.
Despite these large-scale studies, the mt-sDNA test is still a relatively new test and more data are needed on screening outcomes to evaluate its diagnostic accuracy. In this single institutional retrospective study with a limited number of patients, we further confirm a low PPV for colorectal adenocarcinoma with only 3 of 147 patients being found to have CRC (PPV=2.0%). Thus, a positive Cologuard™ test is by no means diagnostic or indicative of CRC. The overall PPV for any precancerous lesion in our cohort is 74.8%, three times higher than the benchmark adenoma detection rate for average-risk screening colonoscopies [16]. As discussed by Eckmann et al [15], the high PPV for any precancerous lesion is likely the result of enrichment for neoplasia in the study population created by screening with the positive Cologuard™ test. In addition, the fact that the endoscopists are aware of positive Cologuard™ results may have increased their diligence during colonoscopy. Therefore, a positive mt-sDNA result appears to have a beneficial impact on the quality of subsequent colonoscopy and the diagnostic yield [17].
There were 34 patients (23.1%) in our cohort who had a positive Cologuard™ test but had no CRC or precancerous lesion detected in the subsequent colonoscopy. One of the categories of biomarkers in the Cologuard™ test is targeting epigenetic changes in the form of gene promoter methylation. DNA methylation has been shown to occur during the normal aging process, which may explain the high false positive rates in patients above the age of 65 [18].
The Cologuard™ test is an acceptable option for patients who are either at higher risk for colonoscopic complications, possibly due to underlying breathing or cardiac issues or for patients who would prefer to undergo a less invasive screening option. It may also draw the attention of younger patients to participate in CRC screening due to its non-invasive nature. In our study, there were 3 patients under the age of 50 who took the Cologuard™ test and had a positive result. The first patient was a 33-year-old male with a history of hemorrhoids and rectal bleeding. Subsequent colonoscopy revealed one tubular adenoma and one SSA/P, both <10 mm in size. The second patient was a 38-year-old male who happened to go to a local health fair where Cologuard™ was performed. His only symptom was occasional streaks of blood on the toilet paper. Subsequent colonoscopy revealed two polyps, which both showed unremarkable colonic mucosa (mucosal folds) on histologic examination. The third patient was a 47-year-old female with obesity, constipation and weight loss. Subsequent colonoscopy revealed 4 hyperplastic polyps, all <10 mm in size.
One of the limitations of this study is its retrospective nature. Because Cologuard™-negative patients less likely undergo colonoscopy in clinical practice, only PPVs for each endpoint could be estimated, without the ability to directly assess other performance characteristics such as sensitivity, specificity, or false negative rates. Selection bias is also present because only Cologuard™-positive patients who also underwent colonoscopy with biopsies/polypectomies were included in this study. Another limitation is small sample size, which is intrinsic to a single institutional study. Nonetheless, our data are convincing and support the conclusions.
Offering a convenient non-invasive and inexpensive screening option with a high sensitivity for curable stage cancer may improve its clinical use and be fully integrated into the CRC screening program. In this study, we evaluated the performance of a positive Cologuard™ test in our patient population. Our study confirm a low PPV for CRC, but a high PPV for precancerous lesions. These data further emphasize the necessity of follow-up diagnostic colonoscopy after a positive Cologuard™ test. Colonoscopy remains to be the gold standard for early CRC detection in the era of molecular medicine.
References
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020; 70: 145-164.
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016; 11: 967-976.
- Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021; 116: 458-479.
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018; 68: 250- 281.
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017; 152: 1217-1237.e3.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016; 315: 2564-2575.
- White A, Thompson TD, White MC, et al. Cancer screening test use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017; 66: 201-206.
- Li B, Gan A, Chen X, et al. Diagnostic performance of DNA hypermethylation markers in peripheral blood for the detection of colorectal cancer: a meta-analysis and systematic review. PLoS One. 2016; 11: e0155095.
- Ahlquist DA. Multi-target stool DNA test: a new high bar for noninvasive screening. Dig Dis Sci. 2015; 60: 623-633.
- FDA approves first non-invasive colorectal cancer screening test. Oncology Times. 2014; 36: 27-29.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014; 370: 1287-1297.
- Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020; 158: 1131-1153.e5.
- Bosch LJW, Melotte V, Mongera S, et al. Multitarget stool DNA test performance in an average-risk colorectal cancer screening population. Am J Gastroenterol. 2019; 114: 1909-1918.
- Cooper GS, Markowitz SD, Chen Z, et al. Performance of multitarget stool DNA testing in African American patients. Cancer. 2018; 124: 3876-3880.
- Eckmann JD, Ebner DW, Bering J, et al. Multitarget stool DNA screening in clinical practice: high positive predictive value for colorectal neoplasia regardless of exposure to previous colonoscopy. Am J Gastroenterol. 2020; 115: 608-615.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015; 81: 31-53.
- Johnson DH, Kisiel JB, Burger KN, et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest Endosc. 2017; 85: 657-665.e1.
- Hashimoto Y, Zumwalt TJ, Goel A. DNA methylation patterns as noninvasive biomarkers and targets of epigenetic therapies in colorectal cancer. Epigenomics. 2016; 8: 685-703.