Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 1
Hepatic expression of glucocorticoid receptors and 11 betahydroxysteroid dehydrogenase type 1 in AIH in pediatric
Mohammed Ahmed Khedr1; Mohamed Abdel Salam El-Guindi1; Nermin Mohammad Adawy1; Doha Maher Taie2; Haidy Mohammed Zakaria3*; Basma Mahmoud Abd-Elaati1
1 Department of Pediatric Hepatology, Gastroenterology and Nutrition, National Liver Institute, Menoufia University, 32511 Shebin El-koom, Menoufia, Egypt.
2 Department of Histopathology, National Liver Institute, Menoufia University, 32511 Shebin El-koom, Menoufia, Egypt.
3 Department of Pediatrics, Berket El-Sba General Hospital, Ministry of Health, 32651 Berket El-Sba, Menoufia, Egypt.
*Corresponding Author : Haidy Mohammed
Zakaria
Department of Pediatrics, Berket El-Sba General
Hospital, Ministry of Health, 32632 Berket El-Sba,
Menoufia, Egypt.
Email: drhaydi2000@gmail.com
Received : Nov 05, 2021
Accepted : Dec 05, 2021
Published : Dec 10, 2021
Archived : www.jjgastro.com
Copyright : © Zakaria HM (2021).
Abstract
Introduction: Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease particularly aggressive in children and progresses rapidly unless immunosuppressive treatment is promptly started.
Aim: We aim to study the hepatic expression of 11β hydroxysteroid dehydrogenase type 1(11β-HSD1) and Glucocorticoid Receptor (GR) in children with AIH.
Patients and methods: This study was conducted on one hundred AIH patients at the Pediatric age group. Liver biopsies of selected cases were retrieved. Biopsy specimens were subjected to; routine histopathological evaluation, 11β-HSD1 and GR immunostaining using antibodies against 11β-HSD1 and GR.
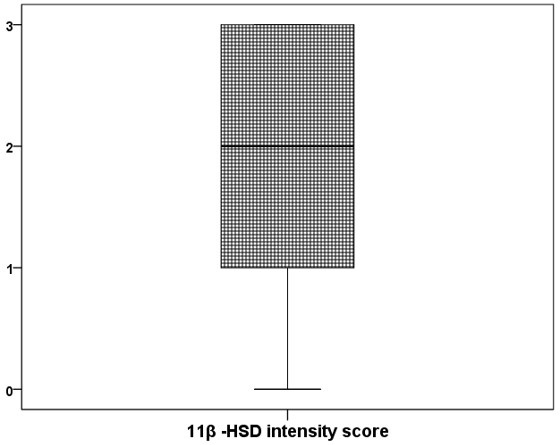
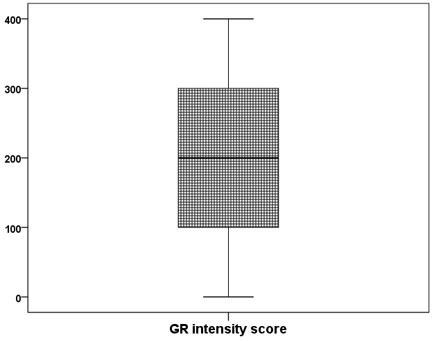
Results: Out of the 100 AIH patients, 39% were males and 61% were females. The mean age at time of diagnosis was 8.03 ± 4.29 years old. The main clinical presentation was recurrent jaundice (38%) followed by manifestations of chronic liver failure (34%). The mean of AIH score was 14.7. The histopathological assessment of the studied group found that; most of cases show mild activity (57%), moderate fibrosis (46%), moderate portal inflammation (58%), moderate IH (41%). The immunohistochemical detection of 11β -HSD and GR of the studied group revealed that the median (25th -75th) expression of 11β –HSD was 2 (1-3) and GR was 200 (100- 300). The GR intensity score has significant positive correlation with intensity of 11β -HSD ( P-value <0.05).
Conclusion: Patients with AIH showing different levels of expression of 11β-HSD1 and GR which may explain the difference in patients’ response to steroid therapy.
Keywords: autoimmune hepatitis; glucocorticoid receptor; 11β hydroxysteroid dehydrogenase.
Citation: Khedr MA, El-Guindi MAS, Adawy NM, Taie DM, Zakaria HM, et al. Hepatic expression of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase type 1 in AIH in pediatric. Japanese J Gastroenterol Res. 2021; 1(9): 1043.
Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory hepatic disease, aggressive in children and can progresses rapidly unless immunosuppressive treatment is adequately started [1]. The treatment aim is induction and maintenance of remission and prevention of subsequent complications, as cirrhosis and hepatocellular carcinoma (HCC) [2]. Glucocorticoids (GCs) are considered the most common anti-inflammatory agents used in treatment of inflammatory and immune diseases. Unfortunately, steroid therapies are accompanied with severe side effects during long-term treatment, which include osteoporosis, hypertension, dyslipidemia and insulin resistance/type 2 diabetes mellitus; so much has been learnt of the molecular mechanisms by which they act [3]. Corticosteroid-related side effects are the most common causes for premature drug withdrawal in AIH that may reach up to 13% of patients. While corticosteroids are used as the first-line therapy for AIH, it has been shown that about 10% of AIH patients are resistant to steroid therapy. These patients have a high risk of disease progression and steroid toxicity, which complicates treatment [4]. 13% of patients are classified as incomplete responders. In these patient alternative strategies must be considered [5]. The perspective tools that allow identification of patients with increased risk of probability of adverse events and lack of efficacy to a particular medication, is particularly desirable and promising [6]. Many factors such as corticosteroid binding globulin, 11β-HSD, and ultimately, the glucocorticoid receptor (GR) affect the local tissue regulation of glucocorticoid availability and action [7]. We aim to study the hepatic expression of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase type 1 in children with AIH.
Patients and methods
This study is retrospective cohort hospital-based study which was conducted on one hundred patients diagnosed as AIH collected from the Pediatric Hepatology, Gastroenterology, and Nutrition department, National Liver Institute- Menoufia University. Medical records related to hospital and outpatient care were analyzed and the data was collected from diagnosed AIH patients. All data were collected from the files of the patients that include: demographic data, presenting clinical features, clinical examination, biochemical parameters, radiological and histological findings, treatment response and outcome. Liver biopsies were retrieved from archives of Pathology Department. Biopsy specimens were fixed in formalin, embedded in paraffin and subjected to; routine histopathological evaluation (stained by hematoxylin and eosin (H&E), Masson’s trichrome, Perls’ Prussian blue stain and Periodic acid-schiff (PAS) stain), 11β hydroxysteroid dehydrogenase type 1(11β-HSD1) and Glucocorticoid Receptor (GR) immunostaining using antibodies against 11β-HSD1 and GR.
Statistical analysis
Data were collected and entered to the computer using SPSS (Statistical Package for Social Science program, version 18, Chicago, Inc, Illinose). Data were entered as categorical or numerical, as appropriate. Quantitative data were expressed as mean± stander deviation (SD) (minimum-maximum) or median (25th -75th) as appropriate. Qualitative data were shown as frequency and percent (%). Mann Whitney U test was done to compare medians of 2 sets of quantitative data non-parametrically distributed. Spearman correlation was used to assess the correlation between quantitative variables. P (probability) value was considered to be of statistical significance if it is ≤ 0.05.
Results
The study included 100 AIH patients 39 (39%) were males and 61 (61%) were females. The mean age at time of diagnosis was 8.03 ± 4.29 years old while the age of first presentation of the disease was 6.8 ± 4 years old. The main clinical presentation of the studied group was recurrent jaundice (38%) followed by manifestations of chronic liver failure (34%). On examination most cases had hepatomegaly (65%) and 45% had splenomegaly. 54% of cases were jaundiced at time of diagnosis (Table 1).
Table 1: Clinical presentations and clinical examination findings of the AIH patients.
Items |
AIH (n=100) |
Clinical presentation |
|
Recurrent jaundice |
38 (38%) |
Chronic liver failure |
34 (34%) |
Picture of acute hepatitis |
28 (28%) |
Elevated enzyme |
24 (24%) |
Incidentally US finding |
23 (23%) |
Fulminant hepatitis |
10 (10%) |
GIT bleeding |
10 (10%) |
Extrahepatic manifestations |
10 (10%) |
Clinical examination |
|
Jaundice at admission |
54 (54%) |
Encephalopathy |
6 (6%) |
Hepatomegaly |
65 (65%) |
Splenomegaly |
45 (45%) |
Ascites |
2 (2%) |
Laboratory parameters of the studied group showed prominent elevation of liver enzymes and bilirubin levels. Most cases had positive ASMA (79%). All cases were negative HCV and HBV (Table 2).
Table 2: Laboratory parameters of the AIH patients.
|
|
|
|
AIH (n=100) |
|
Before treatment |
Mean± SD |
Min- max |
TB (mg/dl) |
5.67± 7.79 |
0.3- 31 |
DB (mg/dl) |
3.7± 5.6 |
0.1-25 |
|||
TP (g/dl) |
7.54± 1.14 |
44473 |
|||
ALB (g/dl) |
3.44±0.7 |
44318 |
|||
AST (U/L) |
424 ± 458 |
19- 2051 |
|||
ALT (U/L) |
365 ± 406 |
13- 2389 |
|||
ALP(U/L) |
298 ± 203 |
43- 1120 |
|||
GGT (U/L) |
116± 193 |
11- 1816 |
|||
PT (sec) |
16.5± 5.3 |
16742 |
|||
INR |
1.6± 0.5 |
1- 4.3 |
|||
HB (g/dl) |
10.9± 1.59 |
42186 |
|||
WBCs (× 103 /mm3) |
7.3± 3.018 |
42430 |
|||
PLT (× 103 /mm3) |
225 ± 117 |
38- 510 |
|||
IgG (g/dl) |
2584 ± 1270 |
702- 6820 |
|||
|
|
Autoantibodies |
|||
|
|
|
ASMA +ve |
79 (79%) |
|
|
|
|
ANA +ve |
26 (26%) |
|
|
|
|
LKM +ve |
9 (9%) |
|
|
|
|
Viral markers (IU/mL) |
||
|
|
|
PCR HCV +ve |
0 (0%) |
|
|
|
|
HCV-Ab +ve |
0 (0%) |
|
|
|
|
PCR HBV +ve |
0 (0%) |
|
TB: total bilirubin; DB: direct bilirubin; TP: total protein; ALB: albumin; WBCs: white blood cells; PLTs: platelets.
The ultrasonographic findings in the studied group revealed presence of hepatomegaly in 54% of cases, splenomegaly in 80%, and ascites was found only in 7% of cases. The most common type of AIH in the studied group was type 1 (75%) followed by seronegative AIH (16%) and Type 2 AIH (9%). The mean of AIH score was 14.7. The most of cases received combined treatment in the form of steroid and azathioprine and only few cases were on steroid monotherapy. Upper gastrointestinal endoscopy was done for 45 cases. It showed that, the most frequent finding was the portal hypertensive gastropathy (PHG) that found in 68.9% of cases followed by dudenopathy and esophageal varices (OV) that were present in 55.5% and 48.9%; respectively. The histopathological assessment of the studied group found that; most of cases show mild activity (57%), moderate fibrosis (46%), moderate portal inflammation (58%), moderate IH (41%), spotty necrosis found in most cases (92%), while confluent necrosis found in 66% of cases (Table 3).
Table 3: Histopathological findings of the AIH patients.
|
AIH (n=100) |
|
Fibrosis stages |
No Fibrosis |
7 (7%) |
Mild Fibrosis |
31 (31%) |
|
Moderate Fibrosis |
46 (46%) |
|
Severe Fibrosis |
16 (16%) |
|
Necroinflammatory activity grades |
No Activity |
2 (2%) |
Mild Activity |
57 (57%) |
|
Moderate Activity |
32 (32%) |
|
Severe Activity |
9(9%) |
|
Portal Inflammation grades |
None |
2 (2%) |
Minimal |
1 (1%) |
|
Mild |
35 (35%) |
|
Moderate |
58 (58%) |
|
Marked |
4 (4%) |
|
Interface hepatitis grades |
None |
15 (15%) |
Mild |
38 (38%) |
|
Moderate |
41 (41%) |
|
Marked |
6 (6%) |
|
Spotty necrosis |
Present |
92 (92%) |
Absent |
8 (8%) |
|
Confluent necrosis |
Present |
66 (66%) |
Absent |
34 (34%) |
|
Table 4: 11β -HSD and GR intensity score in males and females.
|
Male |
Female |
P-value |
(n=39) |
(n=61) |
||
Median (25th -75th) |
Median (25th -75th) |
||
11β -HSD |
1 (1-2) |
2 (1-3) |
0.015 |
GR |
100 (100-200) |
200 (100-300) |
0.031 |
The immunohistochemical detection of 11β –HSD and GR of the studied group revealed that the median (25th-75th) expression of 11β –HSD was 2 (1-3) and GR was 200 (100- 300). The GR intensity score has significant positive correlation with intensity of 11β –HSD (r = 0.369, P-value= <0.0001). On comparing between male and female regarding to 11β –HSD and GR expression, both 11β –HSD and GR were more intense significantly in female than male (P-value < 0.05).
Discussion
Autoimmune hepatitis is a progressive inflammatory hepatopathy, if untreated, leads to end-stage liver disease. The common typical features of AIH are female preponderance, seropositivity for circulating autoantibodies, hypergammaglobulinemia, and a presence of interface hepatitis on histology [8]. Glucocorticoids can effectively relieve AIH, but some patients with this disease are refractory even when GCs are administered. They comprise a group of patients at high risk of disease progression and development of liver failure. These patients are in need of more aggressive immune-modulating treatment, and valuable time may be lost awaiting the absent response to standard treatment [9]. The pharmacological and physiological effects of GCs occurring through the binding to the GR [10]. 11β-hydroxysteroid dehydrogenase type 1 is a crucial enzyme converts inactive cortisone into active hydrocortisone, so it regulating multiple functions of GCs. 11β-HSD1 is widely expressed in tissues and particularly abundant in liver, muscle and fat tissue [11]. Despite that, some studies have investigated the 11β-HSD1 and GR, none was been performed in liver tissues of pediatric nor adult AIH before [12-14]. So, our work had been performed to investigate the hepatic expression of GR and 11β-HSD1 in pediatric AIH.
This study included 100 patients with AIH. Most of AIH patients had received immunosuppressive treatment in form of low-dose prednisolone combined with azathioprine while few patients were treated initially with prednisolone. Autoimmune hepatitis mostly involves patients in second and third decade of life with peak age around 10 years old in children. The mean of the age at time of diagnosis were 8.03 ± 4.29 years old. Our study showed predominance of females (61%). The reason for the high prevalence in females is unclear. Females respond to vaccination, infection and trauma, through increased antibody production and exaggerated T helper 2 predominant immune response [15].
The clinical spectrum in our patients was broad ranging from acute hepatitis with or without liver failure to chronic, unapparent disease with mild, fluctuating increase of liver enzymes, most cases were presented by recurrent jaundice (38%). 75% of AIH patients were type 1. On examination most cases had hepatomegaly (65%) and 45% had splenomegaly. 54% of cases were jaundice at time of diagnosis. Our study is supported by reported observations that prevalence of patients with jaundice as an initial manifestation is high (44-69%). These are considered features of pediatric AIH [17]. On analyzing the laboratory parameters, a wide range due to their different stages of liver disease were present. Elevation of AST and ALT greater than three times the upper limit of normal was observed. The diagnosis of AIH can be challenging with no single pathognomonic feature. IAHG developed and subsequently revised a scoring system to weigh each clinical, laboratory and histological finding at presentation as well as after corticosteroid therap. A pretreatment score of 15 and post treatment score of 17 was considered indicative of ‘definite’ AIH. The mean score of our studied group was 14.87. In the present study, most of cases received combined therapy (steroid+ azathioprine) as standard treatment, based on steroids and azathioprine, is effective in the vast majority of patients [17].
Liver biopsy is necessary for the diagnosis of AIH, also it is used to guide treatment decisions and should be done before starting treatment, unless there are contraindications. Although the histological appearance of AIH is characteristic, there is no morphological feature that is pathognomonic of AIH [18]. In our study, the histopathological findings were quite variable. Most of cases showed moderate portal inflammation (58%), moderate IH (41%), spotty necrosis found in most cases (92%), while confluent necrosis found in 66% of cases. This is agree with [19], who mentioned that, histologic findings that suggest AIH are described as interface hepatitis and/or multilobular hepatitis. Interface hepatitis is characterized by dense inflammatory lymphocytes and plasma cells that infiltrate the portal tracts, swelling of hepatocytes, and necrosis.
Fibrosis and necroinflammation were moderate in most of our cases. This illustrate that AIH is particularly aggressive in children and progresses rapidly unless immunosuppressive treatment is promptly started [20]. In the current study, GR expression found in all cases. The median of its expression was 200 according to H- score. Iguchi et al., 2018 demonstrated, strong and abundant expression of GR in the submaxillary gland, kidney, and retroperitoneum of IgG4-related disease patients. They suggested the abundant expression of glucocorticoid receptor in various types of cells, including resident fibro/ myofibroblasts in IgG4-related disease patients might provide clues to the mechanism of steroid responsiveness in immune mediated disease patients [21]. Although 11β-HSD1 activity has been shown to play an important role in the metabolic actions of glucocorticoids, its role in the immune response has, until recently, remained unclear. We hypothesize that the regulation of cortisol by 11β-HSD1 in liver tissue is a determinant of the pathogenesis of AIH and modulation of this enzyme may provide a novel therapeutic target for the treatment of this common sight-threatening disease. The expression of 11β –HSD in our study had significant positive correlation with the GR intensity score. Although no previous study assessed of 11β-HSD1 expression in AIH disease, several studies assessed the hepatic 11β-HSD1 expression in others liver diseases with conflicting results. Ahmed et al., suggested that in the early stages of nonalcoholic fatty liver disease (NAFLD), with steatosis alone, hepatic 11β-HSD1 activity decreases with the progression to NASH, which is associated with increased 11β-HSD1 levels [22]. Further studies correlating the different expressions of 11β-HSD1 and GR with patients’ response to therapy are needed.
Conclusion
Patients with AIH showing different levels of expression of 11β-HSD1 and GR which may explain the difference in patients response to steroid therapy.
References
- Ferri PM, Ferreira AR, Miranda DM, and Simoes ESAC. Diagnostic criteria for autoimmune hepatitis in children: a challenge for pediatric hepatologists. World J Gastroenterol. 2012; 18: 4470-3.
- Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM, and American Association for the Study of Liver, D: Diagnosis and management of autoimmune hepatitis. Hepatology. 2010; 51: 2193-213.
- Coutinho AE, and Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335: 2-13.
- Ohira H, and Takahashi A. Current trends in the diagnosis and treatment of autoimmune hepatitis in Japan. Hepatol Res. 2012; 42: 131-8.
- Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM, and American Association for the Study of Liver, D: Diagnosis and management of autoimmune hepatitis. Hepatology. 2010; 51: 2193-213.
- Stocco G, De Iudicibus S, Franca R, Addobbati R, and Decorti G. Personalized therapies in pediatric inflammatory and autoimmune diseases. Curr Pharm. 2012; 18: 5766-75.
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012; 1261: 55-63.
- Lowe D, and John S: Autoimmune hepatitis: Appraisal of current treatment guidelines. World J Hepatol. 2018; 10: 911-923.
- Eriksen PL, Kreutzfeldt M, Gronbaek H, Thorsen K, Vang S, Jessen N, and Vilstrup H. Enrichment of Genetic Variants in the Glucocorticoid Receptor Signalling Pathway in Autoimmune Hepatitis with Failure of Standard Treatment. Basic Clin Pharmacol Toxicol. 2017; 121: 189-194.
- Ferrara G, Petrill, MG, Giani T, Marrani E, Filippeschi C, Oranges T, Simonini G, and Cimaz R. Clinical Use and Molecular Action of Corticosteroids in the Pediatric Age. Int J Mol Sci. 2019; 20.
- Xiao W, Lu MH, Rong PF, Zhang HY, Gong J, Peng YQ, Gong HY, and Liu ZG. 11betahydroxysteroid dehydrogenase1 is associated with the activation of hepatic stellate cells in the development of hepatic fibrosis. Mol Med Rep. 2020; 22: 3191-3200.
- Gao L, Wang JF, Xiang M, Fan YC, Zhang ZG, and Wang K. Expression of human glucocorticoid receptor in T lymphocytes in acute-on-chronic hepatitis B liver failure. Dig Dis Sci. 2011; 56: 2605-12.
- Rai T, Monoe K, Kanno Y, Saito H, Takahashi A, Irisawa A, and Ohira H. Expression of human glucocorticoid receptor beta of peripheral blood mononuclear cells in patients with severe autoimmune hepatitis. Fukushima J Med Sci. 2006; 52: 65-70.
- Rai T, Ohira H, Tojo J, Abe K, Yokokawa J, Takiguchi J, Shishido S, and Sato Y. Expression of human glucocorticoid receptor in lymphocytes of patients with autoimmune hepatitis. Hepatol Res. 2004; 29: 148-152.
- Do Socorro Teixeira Moreira Almeida M, da Costa Arcoverde J, Barros Jacobino MN, and Coimbra Neto AR. Male systemic lupus erythematosus, an overlooked diagnosis. Clin Pract. 2011; 1: e103.
- Sogo T, Takahashi A, Inui A, Fujisawa T, Ohira H, Takikawa H, and Japan. AIHSG: Clinical features of pediatric autoimmune hepatitis in Japan: A nationwide survey. Hepatol Res. 2018; 48: 286- 294.
- Terziroli Beretta-Piccoli B, Mieli-Vergani G, and Vergani D: Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017; 23: 6030- 6048.
- Nguyen Canh H, Harada K, Ouchi H, Sato Y, Tsuneyama K, Kage M, Nakano M, et al. Biliary Diseases Study Group of, J: Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol 2017; 70: 961-969.
- Pathak S, and Kamat D: Autoimmune Hepatitis in Children. Pediatr Ann. 2018; 47: e81-e86.
- Ferri PM, Ferreira AR, Miranda DM, and Simoes ESAC: Diagnostic criteria for autoimmune hepatitis in children: a challenge for pediatric hepatologists. World J Gastroenterol. 2012; 18: 4470-3.
- Iguchi T, Takaori K, Mii A, Sato Y, Suzuki Y, Yoshifuji H, Seno H, et al. Glucocorticoid receptor expression in resident and hematopoietic cells in IgG4-related disease. Mod Pathol. 2018; 31: 890- 899.
- Ahmed A, Rabbitt E, Brady T, Brown C, Guest P, Bujalska IJ, Doig C, et al. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS One. 2012; 7: e29531.