Japanese Journal of Gastroenterology Research
Review Article - Open Access, Volume 1
Colonic perforation secondary to fulminant amoebic colitis, review on the subject of a case
Gonzalez-Rodriguez Inés Carolina1*; Salazar-Vargas Alejandra Katherine2
1 Residente del Servicio de Cirugía General del Servicio Autónomo Docente Hospital Central de Maracay, Aragua, Venezuela.
2 Adjunto tipo I del Servicio de Cirugía General del Servicio Autónomo Docente Hospital Central de Maracay, Aragua, Venezuela.
*Corresponding Author : Gonzalez-Rodriguez Inés
Carolina
Residente del Servicio de Cirugía General del Servicio
Autónomo Docente Hospital Central de Maracay,
Aragua, Venezuela.
Email: gonzalezrinesc@gmail.com
Received : Oct 30, 2020
Accepted : Nov 29, 2021
Published : Dec 02, 2021
Archived : www.jjgastro.com
Copyright : © Carolina GRI (2021).
Abstract
A 24-year-old female patient with an appendicular clinic is operated on 10 days prior to admission to a health center where they perform open apical basal appendectomy, whose biopsy reports acute appendicitis in the catarrhal phase, who visits a physician for presenting intestinal content leakage. Through an operative wound, thermal rises, and an emetic episode, she underwent surgery and revealed colonic perforations in its entirety and microabscesses, as well as fecal peritonitis, which warrants subtotal colectomy. Intestinal amebiasis is one of the most frequent causes of parasitic diseases, however, and colonic perforation, due to this entity, is one of the presentations, but it undoubtedly represents high morbidity and mortality from this protozoal infection.
Keywords: amoebic colitis; colonic perforation; intestinal amebiasis; Peritonitis; protozoa.
Citation: Carolina GRI, Katherine SVA. Colonic perforation secondary to fulminant amoebic colitis, review on the subject of a case. Japanese J Gastroenterol Res. 2021; 1(9): 1041.
Introduction
Amebiasis is the infection produced by Entamoeba histolytica, a species of protozoan that parasitizes man, which can live as a commensal in the colon or can invade the intestinal mucosa and have extra-intestinal lesions [2].
The trophozoite of E. histolytica is found in the lumen of the colon or invading the intestinal wall where they reproduce by binary division, these trophozoites eliminate food vacuoles and intracytoplasmic inclusions, which later form the precysts, these acquire a cover and are called cysts immature with nuclei that continue to develop to form the typical tetranucleate cysts. This cystic formation occurs exclusively in the colonic lumen. In human feces, trophozoites, precysts and cysts can be found, the first two die due to the action of external physical agents and, instead of this not happening, gastric secretions destroy them, leaving the cysts that are orally infectious [2].
Approximately 10% of people with E. histolytica in the colon are symptomatic, so the remaining 90% are considered healthy carriers. Not all those with the pathogenic species have disease as this depends on the interaction between the virulence of the parasite and the host’s defenses. The mechanism of mucosal damage to produce colonic ulceration consists of four stages: invasion of the mucosa by trophozoites, virulence factors of amoebae, host resistance, ulcer formation [2].
The initial lesions are the superficial ulceration and its infiltration with the subsequent minimal tissue necrosis, the amoebae actively multiply pass to the muscular mucosae and reach the submucosa where they reproduce and form colonies, destroying the tissues horizontally producing larger perforations, these they are typical “shirt button” injuries. Amoebae stop at the muscular layer, being able to penetrate and spread to the serosa and even perforate it. These ulcerations predominate in the cecum, sigmoid and rectum, these lesions progress and generate excavated ulcers, generating necrosis of large areas of the mucosa, associated with hemorrhage and detachment of fragments of it called fulminant amoebic colitis [1,2,6].
Colonic perforation generally occurs in the cecum, transverse colon and sigmoid, with the passage of intestinal content to the abdominal cavity, it tends to appear as multiple perforations, representing the main cause of death in cases of intestinal amebiasis. Clinically it presents with abdominal pain, diarrhea, tenesmus, vomiting, anorexia and weight loss, in general, bacterial infections are added resulting in a fatal outcome [1,2].
Clinical case
This is a 24-year-old female patient with a 10-day late postoperative history of open appendectomy, performed in a foreign center, who reports onset of current disease 03 days prior to admission when she begins to present leakage of intestinal content, through postoperative wound, concomitant to unquantified thermal rise, 01 emetic episode of bile content; reason for which she goes to the hospital and her admission is decided. Patient refers open basoapical appendectomy, with findings of a cecal appendix, retrocecal 7cm in length x 1 cm in diameter, in catarrhal phase, mesenteric adenopathy, adenopathy in greater omentum, who presents with grade II infection of the operative site according to CDC, which prolongs hospital stay in said hospital, graduating satisfactorily on the seventh day.
Physical Exam: BP: 100/60 mmHg FC: 110 bpm FV: 19 rpm Temp: 39.3o C hemodynamically unstable patient, in fair general conditions, dehydrated, febrile, eupneic, hypotensive, tachycardial, capillary filling greater than 3 seconds Cardiopulmonary: normo-expandable symmetric chest Added, non-auscultated breath sounds present in both lung fields. Rhythmic heart sounds present, auscultated gallop. Abdomen: Flat, evidence of a Rocky-Davis type operative wound in the right iliac fossa, with dehiscent edges with intestinal output through the same hydroaereal sounds present, depressing soft painful on deep superficial palpation in the right iliac fossa. Extremities: symmetrical, mobile eutrophic, without edema, conserved muscular strength. Neurological: Conscious, oriented in time, space and person.
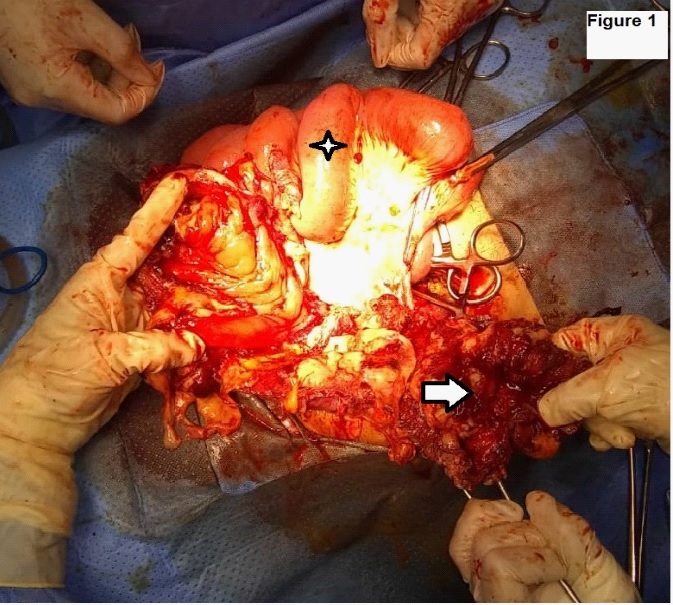
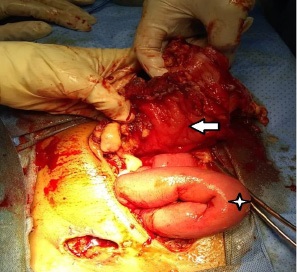
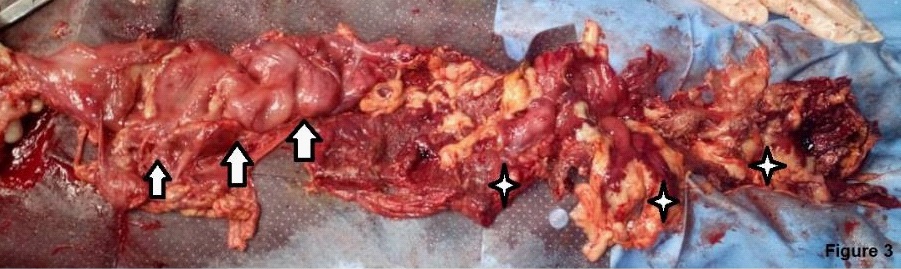
Laboratories: leukocytosis of 13,200 mm3 , with shift to the left, elevation of acute phase reactants. She was taken to the operating table under the context of: Acute surgical abdomen, probable appendicular stump leak, with an exploratory laparotomy plan; Intraoperative findings: (1) Dehiscent operative wound. (2) Poor quality right iliac fossa aponeurosis, with abundant fibrin. (3) 1500 cc of purulent content and free fecaloid in cavity. (4) There is evidence of a solution of continuity in the antimesenteric border from the cecum, ascending colon to the transverse, with exposure of the mucosa, abundant fibrin and fecal content, greater omentum adhered to the colonic mucosa. (5) Ulcerative lesions with rounded edges, from the transverse splenic flexure to the descending colon, with abundant fibrin tissue (Figures 1,2,3) (6). Cecum wall adhered to the sigmoid colon with abundant fibrin tissue, there is no evidence of lesions in the sigmoid or intraperitoneal rectum. (7) Multiple mesenteric adenopathies, 140 cm of thin loop totally undamaged. (8) Rest of intact intra-abdominal organs. Exploratory laparotomy is performed with subtotal colectomy, terminal ileostomy, distal end closure, cavity lavage, and delayed closure.
Patient with immediate and mediate hemodynamically unstable postoperative period BP 80/40 mmHg HR: 135 bpm VF: 28 rpm with dehydration, febrile, eupneic, hypotensive, tachycardia, capillary filling greater than 3 seconds Abdomen: Flat, evidence of operative wound in iliac fossa Right Rocky-Davis type, with confronted edges, median wound with serohaematical output through it, functional ileostomy, water noises present, depressing soft tenderness on superficial palpation in operative wound. It is assessed by the intensive care and infectology service, rotating antimicrobials to carbapenemics of the meropenem type and maintaining nitroimidazoles, associating total parenteral nutrition. Control laboratories: leukocytosis 11,900 mm3 , neutrophilia, anemia 9.1 gr/dL, moderate hypokalemia 2.96 mEq/L, hypoproteinemia 4 gr/dL and hypoalbuminemia 2.1 gr/dL.
Hydroelectrolyte disorders and hemodynamic status are optimized, being taken to the second surgical shift, in which the following findings are evident: (1) Median operative wound with confronted edges. (2) Poor quality aponeurosis. (3) 300 cc of free peritoneal reaction liquid in cavity. (4) Scarce fibrin interases. (5) distention of thin handles and stomach. (6) Rest of intact intra-abdominal organs. An exploratory laparotomy was performed with cavity lavage and definitive closure. During the postoperative period, the patient presented with CDC grade II surgical site infection, who was discharged on the tenth postoperative day, in stable general conditions. Discharge laboratories: count and usual formula, 10 gr/dL of hemoglobin, without hydroelectrolyte disorders. Who to date is in good general condition, being reinstated to work activities, pending restoration of intestinal transit.
Whose anatomopathological study reports colon segment, the serosa is depulid, hemorrhagic with fibrino-purulent exudate. Presents multiple transmural perforations with anfractive and hemorrhagic borders. The antimesenteric section shows the presence of feces, edema and perilesional hemorrhage in the colonic mucosa of the segment. Conclusive findings with:
- Complicated fulminant amebic colitis with areas of transmural necrosis and multiple perforations.
- Transmural hemorrhage, areas of hemorrhagic infarction, edema and congestion of the submucosa.
- Purulent exudative peritonitis.
Discussion
Lösh is the one who describes for the first time in 1875, in Saint Petersburg, a 24-year-old peasant suffering from dysentery, the researcher inoculated feces from the patient in 4 dogs rectally and orally, and producing dysentery in one of them, finding ulcerations in the intestinal mucosa and amoebas in the exudate. The peasant died at 7 months, the necropsy remitted numerous and extensive ulcerative lesions in the mucosa of the colon, later Kartulis 1885-1887 demonstrates the presence of amoebae in 150 autopsies of cases of dysentery, thus affirming that the causal agent of tropical dysentery. It was the amoeba [1,2].
The perforative presentation is one of the rarest of intestinal amebiasis, representing less than 1% of cases, however when this situation occurs it should be considered an abdominal catastrophe, due to the histopathological alterations that occur in the intestinal lumen and its layers generating great destruction of the same, as in the case presented, although multiple perforation is the most common presentation of this type of entity, it is described that the most common perforation sites are blind (55%), recto-sigmoid (25%), hepatic flexure and ascending colon (10%), transverse colon (5%), splenic flexure and descending colon (5%) [2,5,8].
Its clinical expression is variable as it was in our case, which was expressed only in non-dysenteric diarrhea, the literature describes 94-100% from mild diarrhea to intense dysentery. Fever and weight loss due to chronic diarrhea are present in 40% of patients. This conditions the patient to a state of dehydration, malnutrition, and immunosuppression, which in turn translates into an increase in mortality, which consequently implies the perforative condition, associated with a high mortality rate, >50% of cases [1,4,7,9].
Despite finding conservative medical management manifestos in the literature, in view of our case, it was decided to perform surgical resolution, depending on the surgical technique applied, in view of large areas of perforation, as well as the degree of contamination of the non-candidate patient After primary anastomosis and decline in hemodynamic status, it was decided to perform subtotal colectomy, cavity lavage and delayed closure, together with broad-spectrum antimicrobial treatment and nitroimidazoles, following the guidelines in the literature [1,3,6,10].
Currently, the patient is in an ileostomy condition, in stable general conditions with grade I malnutrition, who returned to her usual activities 45 days after discharge, currently awaiting restoration of gastrointestinal transit.
References
- Aristazabal H et al Fulminant amebic colitis. World J Surg 1991;15: 216-21
- Botero D, Restrepo M. Parasitosis humanas 5ta edición, Medellín-Colombia, Corporación para investigaciones biológicas 2012 (ISBN 978-958-9076-77-4 pp 37-75).
- Briceño-Santana M,et al . Colitis amebiana necrosante, presentación de un caso. Rev. chil. infectol. 2020; 37: 599-603.
- Centeno F, et al Manejo de la colitis amibiana fulminante. Rev Mex Coloproctología 1997; 3: 10-14.
- Goel A, Bansal R, Kaur N, Arora P. Isolated sigmoid colon perforation in an unsuspected case of amoebic colitis. Indian J Med Spec 2015; 6: 168-9.
- Gupta S S, et al Acute fulminant necrotizing amoebic colitis: A rare and fatal complication of amoebiasis: a case report. Cases J 2009; 2: 1-4.
- Jiménez-Bobadilla B, et al Perforación colónica por colitis amibiana: informe de un paciente revista Cirujano general 2003; 25: 228-233
- Mahe I, et al Fatal fulminant acute amebic colitis in metropolitan France. Presse Med 2001; 30: 1295-7.
- Ozdogan M, et al Amebic perforation of the colon: Rare and frequently fatal complication. World J Surg 2004; 28: 926-9.
- Suárez Artacho G et al Colitis aguda fulminante causada por amebiasis intestinal. Rev. esp. enferm. dig. 2006; 98: 559-560.