Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 1
Refractory post-band ligation bleeding misdiagnosed as esophageal varix rupture caused by primary biliary cirrhosis: A case report
Yasunori Minami*; Shunsuke Omoto; Masahiro Takita; Yoriaki Komeda; Mamoru Takenaka; Shigenaga Matsui; Naoko Tsuji; Hiroshi Kashida; Masatoshi Kudo
Department of Gastroenterology and Hepatology, Faculty of Medicine, Kindai University, 377-2 Ohno-higashi Osaka-Sayama, Osaka 589-8511, Japan.
*Corresponding Author: Yasunori Minami
Department of Gastroenterology and Hepatology, Faculty of Medicine, Kindai University, 377-2 Ohno-higashi Osaka-Sayama, Osaka 589-8511, Japan.
Tel: +81-72-366-0221 (ext:3149),
Fax: +81-72-367-2880;
Email: minkun@med.kindai.ac.jp
Received : Sep 18, 2021
Accepted : Oct 29, 2021
Published : Nov 03, 2021
Archived : www.jjgastro.com
Copyright : © Minami Y (2021).
Abstract
A 71-year-old woman had refractory post-band ligation esophageal bleeding although emergency EVL was carried out nine times in total. She was originally considered the esophageal variceal hemorrhage in Primary Biliary Cirrhosis (PBC). Through discussion at a multidisciplinary meeting, we could reach an appropriate diagnosis of PBC and incomplete CREST overlap syndrome. It was difficult to distinguish hemorrhagic telangiectasia from bleeding esophageal varices, and argon plasma coagulation safely reduced the risk of rebleeding and necessity of emergency endoscopy. We learned again a valuable lesson that interprofessional communication is essential to overcome diagnostic error and cognitive bias in patients with refractory treatment.
Keywords: cognitive bias; diagnostic error; interprofessional communication; PBC and CREST overlap syndrome; refractory post-band ligation bleeding.
Citation: Minami Y, Omoto S, Takita M, Komeda Y, Takenaka M, et al. Refractory post-band ligation bleeding misdiagnosed as esophageal varix rupture caused by primary biliary cirrhosis: A case report. Japanese J Gastroenterol Res. 2021; 1(6): 1030.
Introduction
Acute gastrointestinal (GI) bleeding is a major medical emergency, and variceal bleeding can be a fatal complication in patients with liver cirrhosis. Upper endoscopy is the mainstay for diagnosis of acute GI bleeding, and variceal bleeding represents 60–65% of bleeding episodes in patients with cirrhosis [1]. Therefore, emergency endoscopy is strongly recommended for patients with hematemesis and liver cirrhosis. To achieve hemostasis, emergent endoscopic treatment should be initiated as soon as possible for a cirrhotic patient who is exsanguinating. Emergency endoscopic variceal ligation (EVL) is a reasonable method for reducing rebleeding and adverse event rates compared with sclerotherapy [2]. However, we may need to change our mindset or treatment strategy on encountering the repeated failure of EVL treatment.
CREST syndrome, also known as limited scleroderma, is a rare connective tissue disease [3]. The acronym "CREST" encompasses the five major features: calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia; however, not all five features are always present. Additionally, CREST syndrome is sometimes complicated by primary biliary cholangitis (PBC) [4]. Therefore, it can be difficult to distinguish hemorrhagic telangiectasia from bleeding esophageal varices in patients with PBC and incomplete CREST overlap syndrome. Our patient, with a long history of morphea, was followed by a rheumatoid connective tissue disease specialist but had not been diagnosed with incomplete CREST overlap syndrome. Herein, we present a case of refractory post-band ligation bleeding misdiagnosed as esophageal varix rupture caused by PBC. Diagnostic errors are a neglected topic in the field of health care and patient safety. This case is presented to discuss our decisive error and raise awareness of potential pitfalls.
Case Report
A 71-year-old woman presented to our department with a 3-day history of passing melena. The patient reported not eating or drinking much that day. She had no complaints of fever, abdominal pain, or weight loss/gain. She was a non-smoker and non-drinker. She had no history of coming into contact with sick people or antibiotic drug use. Her past medical history was notable for localized scleroderma (also known as morphea) and arthritis. Regular medications included bucillamine, beraprost sodium, and celecoxib. At presentation, her vital signs were as follows: temperature, 36.6°C; heart rate, 64 beats/min; respiratory rate, 14 breaths/min; and blood pressure, 111/77 mmHg. On examination, the patient complained of mild discomfort but no acute distress. Her abdomen was soft and nondistended with positive bowel sounds.
Laboratory data indicated normal electrolytes and kidney function. Her hemoglobin was 10.5 g/dL, white blood cell count 7500/mm3, and platelet count 25.8 x 104/ μl. Serum proteins were 7.3 g/dL, and albumin was 4.1 g/dL. Liver function tests showed mild to moderate elevations of serum alkaline phosphatase (ALP) 1182 IU/L and alanine aminotransferase (ALT) 43 IU/L. She was negative for hepatitis B surface antigen (HBs Ag) and hepatitis C antibody (HCV Ab). A test result for anti-centromere antibody was positive; however, negative test results were obtained for anti-mitochondrial antibody (AMA) and anti-neutrophil cytoplasmic antibody (ANCA).
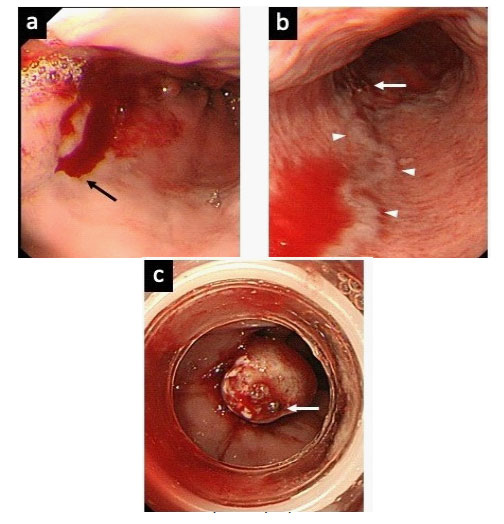
Upper GI tract bleeding was shown endoscopically as oozing from a small varix located in the distal esophagus, and EVL was performed at the bleeding point (Figure 1).
a. Active bleeding (black arrow) near the esophagogastric junction.
b. Temporary hemostasis was achieved (white arrow). A longitudinal vein (arrow heads) might develop with portal hypertension.
c. The bleeding point (white arrow) was ligated in distal esophagus using EVL.
A plain abdominal CT revealed an enlarged liver and spleen without ascites. A needle liver biopsy was performed because of the liver disease diagnosis of uncertain etiology, and the pathological findings of granulomatous and lymphocytic portal inflammation centered on the interlobular bile ducts, and lymphocytic cholangitis supported the diagnosis of AMA-negative PBC in the early stage (Scheuer's stage 1). Originally, we considered the upper GI bleed in cirrhosis to be caused by rupture of an esophageal varix.
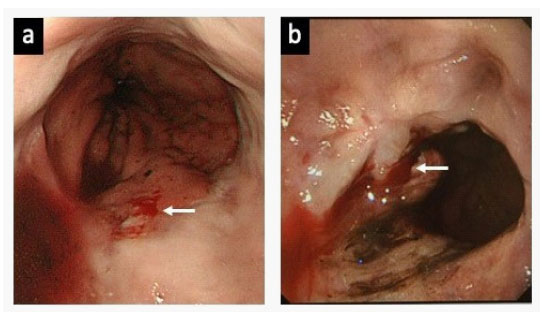
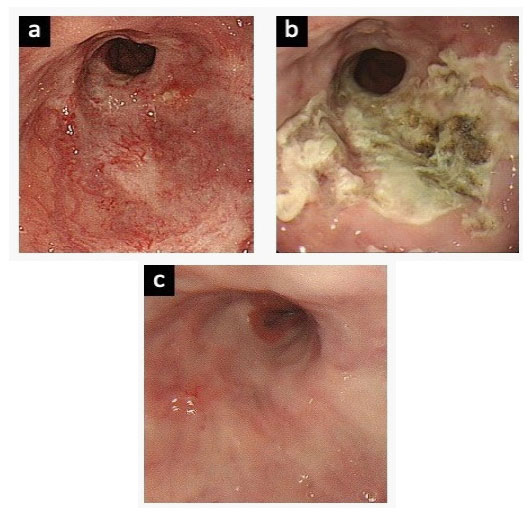
However, the patient was transported to the emergency room 52 days later due to melena, and she received emergency EVL because of distal esophageal bleeding. Subsequently, she complained of haematemesis or melena every 2 to 4 months, due to ruptures of small blood vessels in the distal esophagus (Figure 2) (Table 1). As a result, emergency EVL was carried out a total of nine times, despite additional oral drug treatment including beta-blocker and proton pump inhibitor for prevention variceal bleeding during the clinical course. Finally, she was admitted again two weeks after hospital discharge due to melena (Table 1), and we have discussed the clinical management of this patient at our multidisciplinary GI meeting. She was suspected of having a combination of CREST syndrome and PBC and may have had variceal bleeding rather than hemorrhagic telangiectasia in the distal esophagus. The patient underwent argon plasma coagulation (APC) therapy as scheduled through the working channel of the endoscope under direct visualization using an electrosurgical generator (ERBE ICC 200; ERBE USA, Marietta, Ga) and a 2.3 mm probe (argon gas flow, 1.0-2.0 L/min; power 60 W). There was no active bleeding noted during the gastroscopic examination, and the lesions were treated successfully with APC (Figure 3-a,b). During a follow-up period of more than 36 months, there was no further recurrent bleeding (Figure 3-c).
a. The blood oozing (white arrow) was found near the gastroesophageal junction at the time of 2nd emergency endoscopy.
b. Active bleeding (white arrow) was detected at the time of 5th emergency endoscopy. However, telangiectasia could not be obviously demonstrated.
Table 1: The course of endoscopic treatments.
| Emergency EVL no. | Days after previous EVL | Symptom | Endoscopic treatment |
|---|---|---|---|
| 1 | -- | melena | EVL |
| 2 | 52 | melena | EVL |
| 3 | 51 | melena | EVL |
| 4 | 124 | melena | EVL |
| 5 | 117 | hematemesis | EVL |
| 6 | 85 | hematemesis | EVL |
| 7 | 66 | melena | EVL, APC |
| 8 | 73 | melena | EVL |
| 9 | 16 | melena | EVL |
Note: APC: Argon Plasma Coagulation; EVL: Endoscopic Variceal Ligation; No: Number.
Discussion
CREST syndrome is a rare connective tissue disorder with an unknown/complex pathogenesis and comprises five main features, including telangiectasia [3]. PBC is a chronic and slowly progressive cholestatic liver disease of autoimmune etiology, characterized by injury of the intrahepatic bile ducts, and can eventually lead to liver cirrhosis [5]. Patients with connective tissue diseases often exhibit liver test abnormalities because liver damage can occur from an autoimmune reaction, and systemic sclerosis (SSc) can sometimes overlap with PBC. Literature reviews suggested that 3–50% of PBC patients have SSc, mostly limited cutaneous systemic sclerosis (lcSSc)[6,7]. AMA is positive in more than 90% of PBC patients and 2.5-25% of SSc patients [6]. However, AMA was negative in our patient, along with pathological evidence of PBC, leading to the final diagnosis of AMA-negative PBC. Initially, she had been diagnosed with morphea, an inflammatory skin disorder, and this was the first pitfall, failure to recognize the association between CREST syndrome and PBC.
Variceal rupture is the most common fatal complication of liver cirrhosis due to viral hepatitis, alcohol-related liver disease, nonalcoholic steatohepatitis (NASH), PBC, etc. Variceal band ligation was shown to be better than endoscopic sclerotherapy for bleeding varices and for non-bleeding medium-to-large varices to decrease the risk of bleeding risk [1,2,8]. In addition, ligation is associated with lower rates of rebleeding, fewer complications, and a higher rate of variceal eradication [1,2,8]. The risk of bleeding from varices depends upon a number of factors, including the size, shape, location, and appearance of the varices. Actually, urgent endoscopies could detect small vessels without the red color sign in the distal esophagus in this patient. Unfortunately, even nine emergency EVLs could not prevent upper GI bleeding. The second pitfall was that we regarded recurrent bleeding as variceal rupture associated with PBC. In addition, a stuck mindset and overexpectation of what EVL treatment could achieve effectively led to a vicious cycle, failure-to-rescue. The third pitfall was cognitive bias.
Cognitive bias is increasingly recognized as an important source of medical error. Up to 75% of errors in internal medicine practice are thought to be cognitive in origin, and errors in cognition have been identified in all steps of the diagnostic process [9]. There are many types of cognitive error. Availability error can misestimate the probability of a disease because a memorable experience makes a particular diagnosis more "available" to come to mind. Our diagnostic error of esophageal varix rupture due to PBC could be an example. Moreover, anchoring errors occur when clinicians cling to an initial impression or clinical information, even as conflicting and contradictory data accumulate. We had persisted with ideas in the absence of SSc and presence of esophageal varix rupture. Failures of communication can lead directly to compromised patient care, medical errors, etc. Consequently, nine EVLs were performed by the GI physician on night duty because the patient was always brought to our hospital by ambulance. In the early stage, poor communications, not only between inpatient and outpatient physicians but also between rheumatoid connective tissue disease specialists and us, could be material for our inflection. Fortunately, the multidisciplinary meeting was an opportunity to correct our error. The subsequent diagnosis of PBC and CREST overlap syndrome provided clear prospects and countermeasures. Alternatively, if we had not discussed this case at the multidisciplinary meeting, we might lead to select APC at the tenth emergency EVL due to developing telangiectasia. However, if so, we could not reach a diagnosis of PBC and incomplete CREST overlap syndrome, and diagnostic error could prevent or delay appropriate treatment. Awareness of the potential for cognitive bias does not reduce its occurrence thus traditional error management strategies (e.g. education, training and the enforcement of policies) are unlikely to be valuable. Possible approaches to minimize cognitive errors include minimizing interruptions during the diagnostic process, the use of mindfulness to enhance attention, maximizing team performance/communication and increasing the conspicuity of important information [10]. Again, this made us realize that prevention of physician isolation needs open communication culture, "do not hesitate to communicate if something is of concern".
Conclusion
In conclusion, we describe the case of a patient with refractory post-band ligation esophageal bleeding, and it was difficult to distinguish hemorrhagic telangiectasia from bleeding esophageal varices in this patient with PBC and incomplete CREST overlap syndrome. Argon plasma coagulation for hemorrhagic telangiectasia safely reduced the risk of rebleeding and necessity of emergency endoscopy. We learned a valuable lesson from this patient that interprofessional communication is very effective in identifying our cognitive bias in diagnosis and determining the appropriate treatment.
Declarations
Conflict of interests: The authors have no conflicts of interest to declare.
Patient consent: The patient had given verbal consent for publication of details of the case.
Ethics statement: This study was conducted in compliance with ethical principles outlined in the Declaration of Helsinki and Malaysian Good Clinical Practice Guideline.
References
- Garcia-Tsao G, Sanyal A, Grace N, Carey W and the Practice Guidelines Committee of the American Association for the Study of Liver Diseases, the Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal haemorrhage in cirrhosis. Hepatology. 46:922–938, 2007.
- Cremers I, Ribeiro S. Management of variceal and nonvariceal upper gastrointestinal bleeding in patients with cirrhosis. Therap Adv Gastroenterol. 2014; 7: 206-216.
- LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988; 15: 202-205.
- Ito M, Kojima T, Miyata M, et al. Primary biliary cirrhosis (PBC)-CREST (calcinosis, Raynaud's phenomenon, esophageal dysfunction, sclerodactyly and telangiectasia) overlap syndrome complicated by Sjögren's syndrome and arthritis. Intern Med. 1995; 34: 451-454.
- Kaplan MM, Gerschwin ME. Primary biliary cirrhosis. N Engl J Med. 2005; 353: 1261–1273.
- Rigamonti C, Shand LM, Feudjo M, et al. Clinical features and prognosis of primary biliary cirrhosis associated with systemic sclerosis. Gut. 2006; 55: 388-394.
- Efe C, Torgutalp M, Henriksson I, et al. Extrahepatic autoimmune diseases in primary biliary cholangitis: Prevalence and significance for clinical presentation and disease outcome. J Gastroenterol Hepatol. 2021; 36: 936-942.
- Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019; 25; 364: l536.
- O'Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018; 48: 225-232.
- Antonacci AC, Dechario SP, Antonacci C, et al. Cognitive Bias Impact on Management of Postoperative Complications, Medical Error, and Standard of Care. J Surg Res. 258: 47-53.