Japanese Journal of Gastroenterology Research
Case Report - Open Access, Volume 1
Gastric plexiform fibromyxoma in children: A new case report and review of pediatric literature
Cristina Moglia1; Filippo Parolini1; Vincenzo Villanacci2; Giuseppe di Gaetano3; Dario Moneghini4; Della Casa Domenico3; Giusto Pignata5; Giovanni Boroni1; Daniele Alberti1,6*
1Department of Pediatric Surgery, "Spedali Civili" Children's Hospital, Brescia, Italy.
2Pathology Unit, ASST Spedali Civili, Brescia, Italy.
3Pediatric Radiology Unit, ASST Spedali Civili Brescia, Brescia, Italy.
4Endoscopy, ASST Spedali Civili di Brescia, Brescia, Italy, ASST Spedali Civili Brescia, Brescia, Italy.
5Department of General Surgery II, Spedali Civili, Brescia, Italy.
6Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
*Corresponding Author: Daniele Alberti
Department of Clinical and Experimental Sciences,
University of Brescia, Brescia, Italy.
Email: daniele.alberti@unibs.it
Received : Sep 16, 2021
Accepted : Oct 18, 2021
Published : Oct 25, 2021
Archived : www.jjgastro.com
Copyright : © Alberti D (2021).
Abstract
Introduction: Plexiform Fibromyxoma (PF), also known as Plexiform Angiomyxoid Myofibroblastic Tumour (PAMT) is a rare mesenchymal myxoid tumour originating in different areas of the digestive system. Symptoms and signs are usually elusive: the most common are abdominal discomfort, dyspepsia, nausea and gastrointestinal bleeding with anemia and hematemesis. Imaging tests are useful to identify plexiform fibromyxoma, but only pathology provides the definitive diagnosis and differentiate it from other mesenchymal tumours. PF seems to display a benign behaviour and the only complete surgical removal of the mass represents the gold standard of treatment; no recurrence or metastasis has been described so far. A little more than 100 cases have been described in adulthood with only 14 cases reported up to now in pediatrics.
Case report and review: We report the case of a gastric plexiform fibromyxoma occurred in an 8-year old girl went to our observation with a story of dyspepsia, vomiting, weight loss and anemia. A 4 X 2.5 cm mass was found in the gastric antrum. The tumour was removed by laparoscopic antrectomy and the pathological exam was consistent with PF. A detailed review of PF pediatric literature is also provided.
Keywords: plexiform fibromyxoma; gastric tumour; pediatrics; laparoscopic gastric resection.
Citation:Alberti D, Moglia C, Parolini F, Villanacci V, Gaetano GD, et al. Gastric plexiform fibromyxoma in children: A new case report and review of pediatric literature. Japanese J Gastroenterol Res. 2021; 1(6): 1028.
Introduction
Plexiform Fibromyxoma (PF), also known as Plexiform Angiomyxoid Myofibroblastic Tumour (PAMT), is a rare gastrointestinal mesenchymal myxoid tumour first reported by Takahashi et al. in 2007 [1]. Adopting the name of plexiform fibromyxoma, this tumour is described as a distinct entity among mesenchymal tumours in WHO Classification of Tumours of the Digestive System (2) as it displays peculiar multinodular plexiform patterns [3], bland spindle cells, myxoid or fibromyxoid stroma with an arborizing vasculature [4,5] and specific immunohistochemical characteristics. PF predominantly arises from the muscularis propria of the gastric antrum [6] with very few cases found in esophagus [7], duodenum [8], gallbladder [9] and colon [10]. The prevalence is similar in both genders with a male-to-female ratio approximately 1:1 [3] with a wide age range at diagnosis from childhood to eldery [5]. The most frequent symptoms are epigastric discomfort or abdominal pain, dyspepsia, nausea, vomiting, weight loss, hematemesis and anemia. Rarely, the tumour is incidentally found during endoscopy or at radiological images [5]. Plexiform fibromyxoma is considered to be a benign tumour, as it presents low proliferation index and poor mitotic activity; metastases and recurrence following complete excision have not been described so far. We report a new case of gastric antrum PF occurred in an 8-year-old female, operated with a laparoscopic technique, which can be added to the only 14 previous reported pediatric cases to improve our knowledge about this rare tumour. A detailed review of PF pediatric literature is also provided.
Case report
Symptoms and signs: An 8-year-old girl came to our emergency department because of lasting and bothersome nausea with occasional episodes of vomiting in the last past year. These symptoms have been increasing in the past five months with appearance of weight loss, blocking of height gain and constipation. Her past history was unremarkable. Physical examination of the abdomen was normal. Blood examination were normal, except for low haemoglobin values and low mean corpuscular volume (Hb 7.7 g/dL; MCV 70,4fL).
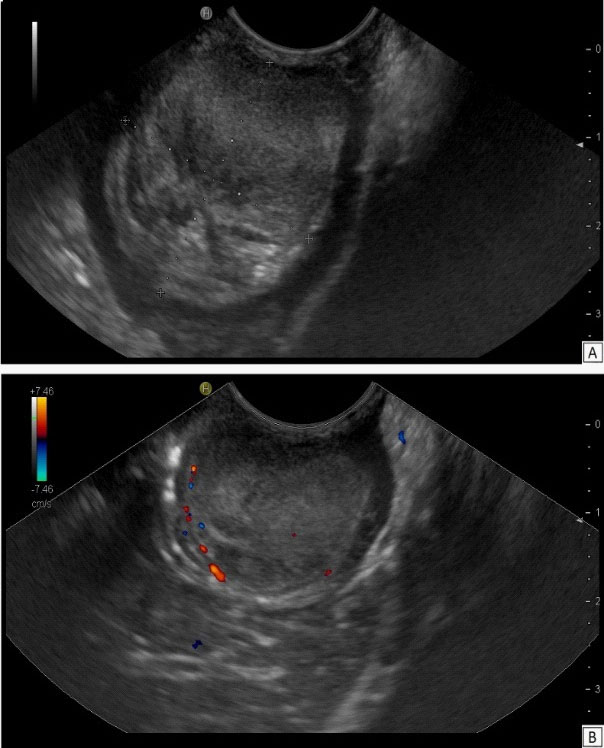
Diagnostic assessment: Abdominal Ultrasound (US) showed a 3,5 X 3 cm solid mass originating within the gastric wall. The tumour was well circumscribed, homogeneously hypoechoic, poorly vascularised at Color-Doppler evaluation and because of its proximity to the pylorus it caused a partial gastric outlet obstruction.
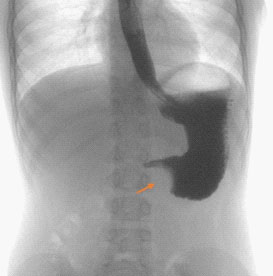
Upper gastrointestinal tract series showed a large, round filling defect of 3,3 cm in diameter, with regular shapes and located between the gastric antrum and pylorus (Figure 1).
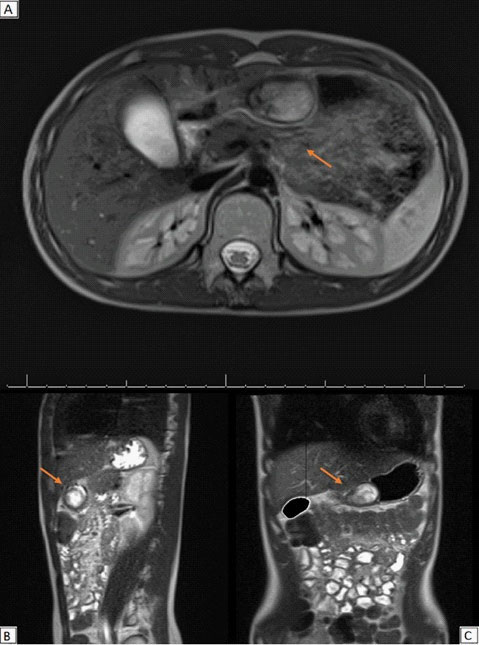
MRI scans of the abdomen confirmed a solid oval mass of 4 X 2,5 cm, with regular profiles (Figure 2). The mass arose from the pyloric antrum with upstream stomach dilated by fluid. It showed an hyperintense signal in T2-weighted sequences with no diffusion restriction to the ADC map and homogenous post-contrast enhancement. Imaging was suggestive of mesenchymal tumour.
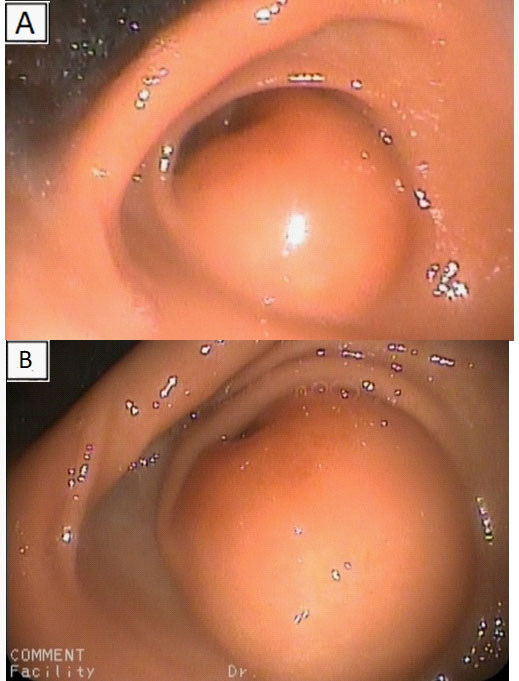
At Endoscopic Ultrasound (EUS) a large bulging lesion about 4 X 2,5 cm in size, located at the anterior wall of the antrum, with a normal-appearing overlying mucosa was detected (Figure 3). The mass, arising from the third layer of the gastric wall, presented an isoechoic lesion, with small hypoechoic foci in the deeper part, without involvement of the muscolaris propria of the stomach and with a weak Color-Doppler signal at the peripheral inner margin (Figure 4). A fine needle aspiration was performed. The tumor, with valve effect, almost completely obstructed the entrance to the pylotus.
Cytological features: Microscopic examination of the cytological specimen revealed aggregates of spindle cells without signs of atypia and no abnormal mitotic activity. The cytomorphological features suggested a spindle cells neoplasm of mesenchymal nature.
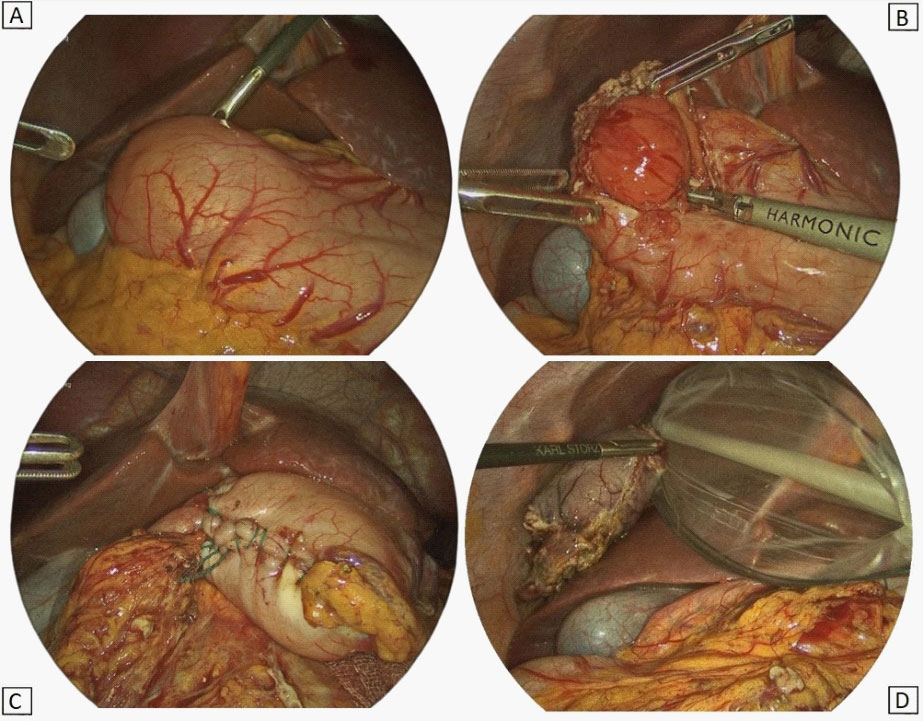
Surgery: Two days after the admission, the patient underwent to surgery. Laparoscopy confirmed the origin of the mass from the gastric antrum. As enucleation of the tumour was not feasible, a laparoscopic pylorus sparing antrectomy with a gastro-pyloric anastomosis was performed (Figure 5).
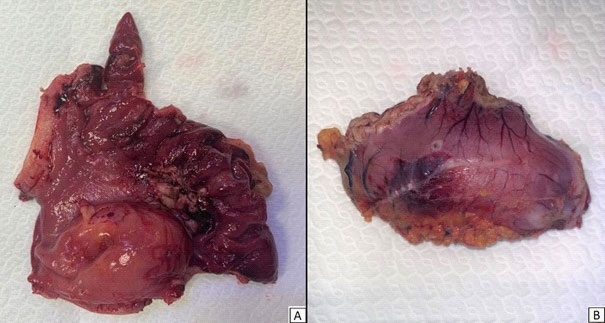
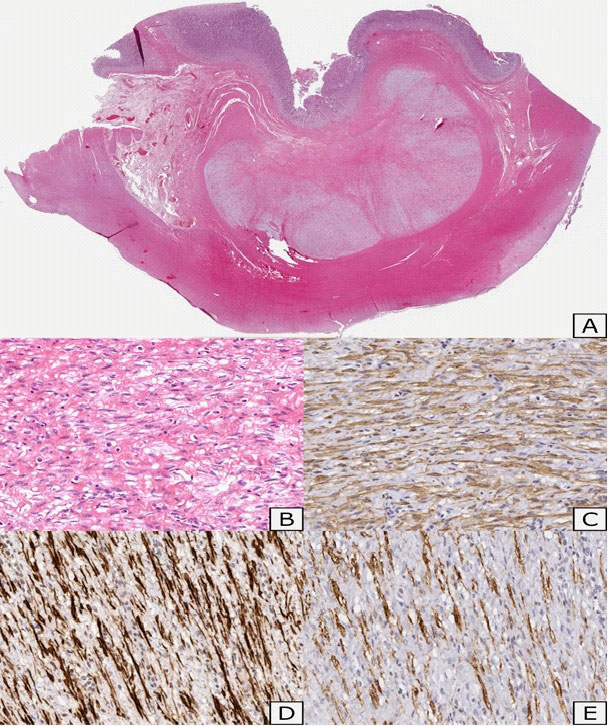
Histopathological features: Gross examination of the surgical specimen showed a nodular lesion of 4 X 2,5 cm, macroscopically limited to the submucosal layer, with greyish appearance on cut sections (Figure 6). Microscopically, the lesion showed a multilobulated and plexiform growth pattern. It was formed by spindle cells with no cyto-nuclear atypia, embedded in a myxoid matrix with fibrous features. In some areas, the tumour had poor cellularity while in others it was more abundant and disorganized with a vascular component. Focal inflammatory infiltrate was detected too. Immunostaining for muscle actin and desmin showed strong and diffuse positivity, while caldesmon staining was less intense (Figure 7). Tumour cells were negative for cytokeratins, CAM 5.2, S100, STAT6, MUC4, CD34, CD117, DOG1, p16, CD10. Proliferation index (percentage of Ki67 positive cells), was less than 5%. Fluorescence in situ hybridization (FISH) assay was negative for MDM2 amplification, These findings were consistent with PF.
Follow-up
The post-operative course was uneventful. Three months after surgery a gastroduodenoscopy was performed. The exam showed hypo-continent cardias and normal gastric mucosa. The histological examination of the biopsies showed of mild esophagitis and active chronic gastritis. At 1-year follow-up, the patient was well, normally growing and with normal abdominal US.
Literature review
The PubMed database was searched for original studies on PF published since 1990, involving patients younger than 18 years of age. Eligible study designs were case reports, case series and reviews. We omitted reports in which abstracts indicated an adult population (> 18 years) and improper reporting of the diagnosis and treatment methods. We then evaluated the full text of the selected articles and consider only where that diagnosis was confirmed by authors. The date of the last search was September 2021.
Results
The initial PubMed search yielded 89 potentially relevant studies. Eventually, 12 eligible articles met the inclusion criteria, involving 14 children with PF (Table 1) for a total of 15 pediatric cases including ours. All selected studies were case reports (class of evidence Ⅲ and rating scale of evidence E). Baseline demographic, clinical characteristics and surgical treatment of the 15 patients are shown in Table 1. Median patient's age was 12 years (range 5 -18 years); 9 patients were female (60%). The most frequent reported symptoms were abdominal discomfort (36%) anemia (29%) and gastrointestinal bleeding (29%). Ulcerative lesions of the tumour were observed in 6 patients (55%) and 3 of them had contextual gastrointestinal bleeding. In 13 cases (87%) the location of the tumour was within the stomach, always involving the gastric antrum. In 1 patient presenting with thoracic pain, shortness of breath and numbness of fingers (Table 1, pts no. 4) the tumour was founded in the middle-third of the esophagus and caused tracheal compression. Median tumour size at diagnosis was 5,25 cm (range 3-15 cm). All patient underwent to surgical treatment, but only in 3 cases, including ours, a minimally invasive technique was used.
Table 1: Demographics, clinical characteristics and treatment of 15th PF pediatric patients.
| No | Age | Sex | Symptoms | Tumor Location | Size (cm) | Ulceration | Treatment | tumour Recurrence | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | F | Vomiting, diarrhea and evident abdominal mass | Gastric antrum, pylorus and duodenal bulb | 15 X 11 X 8 | N | Tumour Resection | No | [3] |
| 2 | 16 | F | Hematemesis | Gastric antrum and pylorus | 10 X 9 X 6 | Y | Distal Gastrectomy | No | [3] |
| 3 | 12 | M | Gastrointestinal bleeding | Gastric Antrum | n.d. | n.d. | Partial Gastrectomy | No | [15] |
| 4 | 11 | F | Anemia | Gastric antrum and pylorus | 3,5 | Y | Laparoscopic distal Gastrectomy | No | [7] |
| 5 | 16 | F | Chest pain,shortness of breath and numbness of finger | Esophagus at the level of the carina | 3,2 | N | Thoracoscopic tumour Resection | No | [7] |
| 6 | 9 | F | Abdominal pain, nausea, vomiting and weight loss | Gastric antrum, posterior | 5 | Y | Laparotomic anterior gastrotomy with tumour resection | No | [16] |
| 7 | 18 | F | n.d. | n.d. | 4,5 X 3,5 X 2,7 | n.d. | n.d. | No | [17] |
| 8 | 11 | M | Abdominal pain | Gastric antrum | n.d. | n.d. | Partial Gastrectomy | No | [18] |
| 9 | 11 | M | Right epigastric discomfort with episodic pain | Gastric pylorus | 17 X 10,5 X 5 | N | Partial Gastrectomy | No | [19] |
| 10 | 16 | F | Anemia and nausea | Gastric antrum, anterior wall | 6,5 | Y | Distal Gastrectomy and retrocolic gastrojejunostomy (Billroth II) | No | [20] |
| 11 | 14 | M | Anemia, fatigue and gastric pain | Gastric antrum | 5,0 | N | Partial Gastrectomy | No | [21] |
| 12 | 14 | F | Abdominal pain, hematemesis | Gastric antrum | 5,5 | Y | Partial Gastrectomy | No | [5] |
| 13 | 5 | M | Pale complexion | Gastric antrum | 8,2 X 7,5 X 5,5 | n.d. | Distal gastrectomy | No | [22] |
| 14 | 16 | M | Hematemesis, weight loss | Gastric cardia near gastroesophageal junction | 5 | Y | Laparotomic wedge resection | No | [23] |
| 15 | 8 | F | Vomiting, anemia, weight loss and constipation | Gastric antrum, anterior | 4,0 | N | Laparoscopic antrectomy | No | our case |
Discussion
Plexiform fibromyxoma is a very rare mesenchymal tumour; in a recent review in 2019, Su et al. collected 121 cases of PF. In the past the prevalence was consider similar in both genders with a female-to-male ratio 1:1 [5], but in a recent review Su et al. found a female predominance (57% of patients) and a range ages from 5 to 81 years (mean age 43 years, median age 46 years) [11]. It is predominantly a tumour of adulthood with an adult-to-child ratio 8:1 according to reported cases and up to now only 14 pediatric cases have been reported.
Symptoms and signs vary from abdominal discomfort or pain, dyspepsia, weight-loss, nausea and vomiting (secondary to pyloric obstruction), to gastrointestinal bleeding with anemia and hematemesis (due to ulceration and invasion of the mucosa). PF is a lobulated submucosal or transmural mass, originating from the muscularis propria [12], usually localized in the gastric antrum, but it has been found in other gastric and extra-gastric areas. The size of the tumour at diagnosis can be very different from 1,5 cm up to 15 cm in longest axis (average size 4,8 cm, median size 4 cm).
As PF displays common clinical and radiological findings with other mesenchymal tumours like GISTs, neuronal tumours, smooth muscle tumours and fibroblastic tumours [3], pre-operative diagnosis is unreliable. Indeed, histological features are typical and show a plexiform growth pattern with multiple nodules with spindle-cells embedded in an abundant myxoid or fibromyxoid stroma [13]. Mitoses are rare and cells have no signs of atypia. Vascular invasion is reported in literature suggesting that intravascular tumour spread is possible [13]. Immunohistochemically, a diffuse positive reaction to vimentin, Smooth Muscle Actin (SMA) and Muscle Specific Actin (MSA) is founded, indicating the fibroblastic, myofibroblastic and smooth muscle cell natures of PF [11]. Focal expression of desmin and caldesmon has been found, suggesting PF is able to smooth muscle differentiation [14].
Regardless patients' age, PF displays a benign clinical behaviour and no recurrence, metastasis or tumour-related deaths have been reported in literature so far. The positive prognosis is also confirmed by the absence or low degree of atypia and low mitotic index (Ki67), with surgery being limited only to tumour excision with no regional lymphadenectomy performed [13]. However, due to scanty literature with short follow-up, it is not possible to exclude with certainty that the tumour could express malignant features in the long run. Analysing the pediatric literature, 15 cases including ours have been reported up to now. In 14 cases, PF was localized in the gastric antrum or pylorus and the only case of tumour localized in the esophagus was detected in a 16 years old girl (Table 1, pts no. 5). Biological, clinical and radiological features of pediatric PF at diagnosis are similar to adult ones except for tumour size, which was larger in children. This difference is probably explained by age-related higher tumour cell proliferation and lower frequency of incidental diagnosis in childhood [5]. Ulceration of the mucosa was frequently reported and that was always associated with digestive symptoms. As in adults, for whom the mainstream treatment is the surgical removal of the tumor [11], all children underwent surgery, with a minimally invasive approach only in 3 cases (Pts no. 4, 5 and 15). Even in children, partial gastric resection was the most performed surgery, but differently from adults, none of the pediatric patients underwent to endoscopic PF removal, probably due to the size of the tumor at diagnosis. In our experience a safe and complete tumor resection has been accomplished laparoscopically, with the well-known advantages of this technique [4] and we suggest it might be considered the gold standard of the treatment, even in pediatrics. Eventually, as now data have been reported about long term follow-up and postoperative quality of life of the pediatric patients, a scheduled control throughout adulthood is mandatory.
Conclusion
Despite plexiform fibromyxoma is extremely uncommon in the pediatric settings, it should be included in the differential diagnosis of gastric tumours even in childhood and teen ages, here we have reported the 15th pediatric case. PF definitive diagnosis is exclusively histological due to the difficulty of distinguishing it from other mesenchymal tumours only by imaging assessment. The benign behaviour of the tumour justifies a surgical approach, limited only to tumour excision with no excessive free resection margins and no need of regional lymphadenectomy. Nevertheless, a long run follow-up is mandatory at least in pediatrics. In our experience a safe and complete resection has been accomplished laparoscopically, with the well-known advantages of the mini-invasive surgery.
References
- Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2007; 31: 724-8.
- Board, WHO Classification of Tumours Editorial, Digestive System Tumours, 5th edition. 2019.
- Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: A distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009; 33: 1624-32.
- 4. Nagahisa Y, Itou T, Okita C, Yamakawa T, Chen K, Kouda Y, Hashida K. Laparoscopic and Endoscopic Cooperative Surgery for Plexiform Angiomyxoid Myofibroblastic Tumor. Case Rep Gastroenterol. 2016; 10: 302-7.
- Fukazawa M, Koga H, Hiroshige S, Matsumoto T, Nakazono Y, Yoshikawa Y. Pediatric plexiform fibromyxoma: A PRISMA-compliant systematic literature review. Medicine (Baltimore). 2019; 98: e14186.
- Lai J, Kresak JL, Cao D, Zhang D, Zhang S, Leon ME, Shenoy A, Liu W, Trevino J, Starostik P, Gonzalo DH, Wang H, Liu X, Fan X. Gastric Plexiform Fibromyxoma: A Great Mimic of Gastrointestinal Stromal Tumor (GIST) and Diagnostic Pitfalls. J Surg Res. 2019; 239: 76-82.
- Duckworth LV, Gonzalez RS, Martelli M, Liu C, Coffin CM, Reith JD. Plexiform fibromyxoma: report of two pediatric cases and review of the literature. Pediatr Dev Pathol. 2014; 17: 21-7.
- Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity. BMJ Case Rep. 2015; 2015: bcr2015210004.
- Fassan M, Salmaso R, Saraggi D, Alaggio R, Guido M, Balsamo L, Carniato S, Gruppo M, Ninfo V, Bardini R, Rugge M. Plexiform fibromyxoma of the gallbladder. Pathologica. 2015; 107: 181-4.
- Daum O, Jirasek T, Grossmann P, Mukensnabl P, Michal M. Plexiform fibroma of the colon. Appl Immunohistochem Mol Morphol. 2010; 18: 483-4.
- Su H A, Yen HH, Chen CJ. An Update on Clinicopathological and Molecular Features of Plexiform Fibromyxoma. Canadian journal of gastroenterology & hepatology. 2019; 3960920.
- Jonaitis L, Kiudelis M, Slepavicius P, Poskienė L, Kupcinskas L. Plexiform angiomyxoid myofibroblastic tumor of stomach: A rare case. World journal of gastrointestinal endoscopy. 2016; 8: 674–678.
- Arslan ME, Li H, Fu Z, Jennings TA, Lee H. Plexiform fibromyxoma: Review of rare mesenchymal gastric neoplasm and its differential diagnosis. World journal of gastrointestinal oncology. 2021; 13: 409–423.
- Quero G, Musarra T, Carrato A, Fici M, Martini M, et al. Unusual focal keratin expression in plexiform angiomyxoid myofibroblastic tumor: A case report and review of the literature. Medicine. 2016; 95: e4207.
- Wang FH, Chen ZR, Niu HL, Zeng RX, Xia JQ. Zhonghua bing li xue za zhi = Chinese journal of pathology. 2012; 41: 190–191.
- MW. Morris, L Sullivan DE. Sawaya MA. Steiner, MJ. Nowicki. "Gastric plexiform fbromyxoma tumor in a child – case report and review of the literature. Journal of Pediatric Surgery Case Reports. 2016; 4: 38–41.
- Spans L, Fletcher CD, Antonescu CR, Rouquette A, Coindre JM, et al. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. The Journal of pathology. 2016; 239: 335–343.
- L Li C. Han F Xu, W Wu, W Cui. Plexiform fbromyxoma: a newly recognized mesenchymal tumor. Chinese Journal of Diagnostic Pathology. 2016; 11: 825–827.
- Liang L, Fanzong L, Peixi Z, Cuihong H. Plexiform angiomyxoid myofibroblastic tumor of the stomach: A case report. Diagnostic cytopathology. 2017; 45: 55–58.
- Szurian K, Till H, Amerstorfer E, Hinteregger N, Mischinger HJ, et al. Rarity among benign gastric tumors: Plexiform fibromyxoma - Report of two cases. World journal of gastroenterology. 2017; 23: 5817–5822.
- Djurić Z, Stojšić Z, Radulović S, Janković R, Milovanović IS. Plexiform Fibromyxoma: A Rare Benign Gastric Tumor. Journal of pediatric gastroenterology and nutrition. 2019; 68: e67.
- Li J, Gao H, Lv M, Ma Y, Wang M. Gastric plexiform fibromyxoma: A rare case in a 5-year-old male. Pediatric blood & cancer. 2019; 66: e27638.
- Nasralla A, Alwabari M, Alsaif O, Amr SS. Gastric Plexiform Fibromyxoma Arising in the Cardia in an Adolescent Male: A Rare Tumor with an Unusual Location. Case Rep Surg. 2020; 2020: 9037960.