Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 1
The long-term outcome of gastrointestinal stromal tumors treatment
Putticha Keawmanee; Wisit Kasetsermwiriya*; Pakkapol Sukhvibul; Suphakarn Techapongsatorn; Natawan Sirivongs; Satit Srimontayamas
Department of Surgery, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Thailand.
*Corresponding Author: Wisit Kasetsermwiriya
Department of Surgery, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, 681 Samsen road, Dusit, Bangkok, 10330, Thailand.
Tel: +66-244-3282
Email: wisit@nmu.ac.th
Received : Aug 05, 2021
Accepted : Sep 01, 2021
Published : Sep 10, 2021
Archived : www.jjgastro.com
Copyright : © Kasetsermwiriya W (2021).
Abstract
Background: To evaluate the overall survival, disease free survival, clinicopathological characteristics and prognostic factors of patients with gastrointestinal stromal tumors (GISTs) who were treated at Faculty of Medicine Vajira Hospital.
Methods: A retrospective review data of 39 GISTs patients who underwent surgery at Vajira hospital from 2007 to 2011 was performed. The patient characteristics, overall survival, disease-free survival of patients and prognostic factors were evaluated.
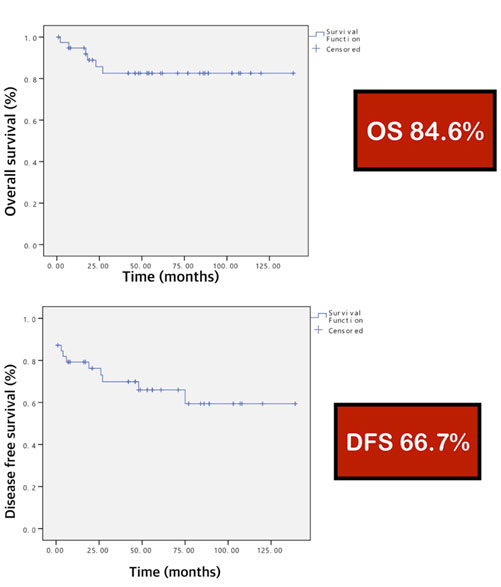
Results: The median age of patients was 60 years (22-90 years), 54% were female and the main presenting symptom was abdominal pain (33%). Stomach (62%) and small intestine (30%) were the most common locations of tumor respectively. Complete resections (R0) were performed in 31 patients (80% ). All patients (100%) were positive for CD117 and 84.6% for CD34. According to the Armed Forces Institute of Pathology criteria, 56.4% of patients were characterized as a high risk. With a median follow-up time of 46 months (1-139 months). The overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively. Two patients received Imatinib in neoadjuvant setting while 10 patients for disease control. Eight patients (20.5%) developed recurrences. R0 resection, mitotic figure and location of tumor were the significant prognostic factors.
Conclusions: The overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively. R0 resection, mitotic figure and location of tumor were the significant prognostic factors.
Keywords: gastrointestinal stromal tumor; gists; prognostic factors; survival.
Citation: Keawmanee P, Kasetsermwiriya W, Sukhvibul P, Techapongsatorn S, Sirivongs N, Srimontayamas S. The long-term outcome of gastrointestinal stromal tumors treatment. Japanese J Gastroenterol Res. 2021; 1(4): 1016.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, accounting for 80% of all digestive mesenchymal tumors. It is widely accepted that GISTs arise from the interstitial cells of Cajal, and the term 'stromal tumor' was first introduced by Mazur and Clark in 1983 [1]. The incidence of GISTs has been reported ranging between 11 and 15 per million annually [2-4] and 60% of GISTs are located in the stomach, 30% in the jejunum or ileum, 5% in the duodenum and 4% in the colorectal. Extra gastrointestinal GISTs have been reported in the liver, omentum and mesentery [5].
The standard curative treatment of GIST is complete surgical resection with negative margin. The risk s of recurrence and metastasis depend on various factors including tumor location, mitotic rate, tumor size, and tumor rupture. [6] Armed Forces Institute of Pathology (AFIP) risk classification criteria are commonly used to predict the prognosis of GISTs [7-8]. Previous reports have shown 5-year survival rate of GISTs varies from 48% to 92.1% [1-9].
The objectives of this study are to evaluate the overall survival, progression free survival, clinicopathological characteristics and prognostic factors affecting patient's survival with gastroKeywords intestinal stromal tumors (GISTs) at Faculty of Medicine Vajira Hospital.
Material and methods
From January 2007 to December 2011, 45 patients diagnosed with GIST and treated at Vajira hospital. Patients were followed up and enrolled into the database of the department of Surgery. Demographic data of the patients including clinical presentation, extent of disease and the prior treatment before patients were treated at Vajira Hospital were recorded. Tumors were categorized as primary, metastatic, or local recurrent disease. The histologic diagnosis of all tumors was confirmed by members of the pathology department at Vajira Hospital. There were 3 patients who were under 18 years old, 2 patients did not receive surgery and 1 patient had surgery at other hospital, these were excluded from this study. Finally, total of 39 patients were enrolled for analysis.
Tumor size was recorded by using the largest diameter in any dimension of the primary tumor and was stratified as <10 cm, or >10 cm. Margins of resected specimens were analyzed for the presence of microscopic disease. The surgeons at Vajira Hospital share a treatment philosophy of GIST which emphasizes the complete gross removal of the tumor. Resections are classified as incomplete or complete. Incomplete resection is considered when the tumor is unresectable at exploration or gross residual of the disease is present after resection. Complete resection is considered when all gross tumor were resected, regardless of microscopic margins. Resection of metastases is performed in selected patients in whom the primary tumor is controlled.
Statistical analysis
Categorized variables were summarized as percentage. Continuous variables were summarized as median. Analysis of cumulative disease free survival and cumulative survival was performed by Kaplan meier analysis. A log rank test was used for univariate analysis. P value <0.05 were considered statistically significant. All statistical analyses were perform using SPSS software version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Clinicopathological characteristics
As shown in Table 1 and 2, total of 39 patients with GISTs were analyzed in the present study. Of all the patients, 18 (46%) were male, and 21 (54%) were female. The median age of the patients was 60 (range, 22–90) years at the time of GIST detection. Stomach was the most common site, accounting for 61% of cases, followed by the small intestine (31%), colorectum and anus (5%), and retroperitoneum (3%). Abdominal pain, abdominal mass and gastrointestinal bleeding were the main presenting symptoms, reported in 33, 23 and 18% of the patients, respectively. The median tumor size was 7 (0.4–60) cm. All of which, 25 (64.1%) <10 cm, 14(35.9%) greater than 10cm. All patients were tested positive for CD117 and 86.4% for CD 34. Most patients, 23 case (59%) had tumor of low mitotic rate (<5 mitoses per 50 HPFs). The histological subtypes included spindle 89.7%, epithelioid or mixed 10.3%. When classified according to Armed Forces Institute of Pathology classification, 2 patients (5.1%) were classified into the very low-risk group, 5 (12.8%) into the low-risk group, 10 (25.6%) into the intermediate-risk group, and 22 (56.4%) into the high-risk group.
Oncologic outcome and recurrence
Twenty nine patients (74%) underwent open surgery and 10 patients (26%) underwent laparoscopic surgery. Complete resection (R0) was accomplished in 31 case (80%), R2 or tumor rupture was 12.3% and R1 resection 7.7%. Two patients (0.78%) had postoperative intra-abdominal collection complications. The average length of stay was 11.5 days (3–73 day).
Imatinib was administered in 10 patients who had incomplete resection, tumor rupture, recurrence or metastasis as an adjuvant therapy, 2 of which were treated as neoadjuvant imatinib therapy. Imatinib were given for a median time of 53 months (19-87 months) at a daily dose of 400 mg. 1 patient developed abnormal liver function. The remaining high-risk cases or cases that should be receiving neoadjuvant therapy but refused imatinib were mainly due to the high cost of the treatment, which was not covered by health insurance or private funding.
In survival analysis based on the 39 GISTs patients, The overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively (Figure 1). A total of 8 patients developed liver metastases or local recurrence during follow-up show as Table 3. The results of the univariate analysis of potential prognostic factors are presented in Table 4. Complete resection (R0), tumor location at stomach and less mitotic figure (<5 mitoses per 50 HPFs) are contributed toward good survival (OS and DFS; P<0.05). However, sex, histological cell type , tumor size and risk category had no significant (OS and DFS; P>0.05).
Table 1: Patients' characteristics.
| Clinical presentation | |
|---|---|
| GI bleeding | 7 (18) |
| Abdominal pain | 13 (33) |
| Anemia | 3 (8) |
| Weight loss | 1 (3) |
| Abdominal mass | 9 (23) |
| Asymptomatic | 6 (15) |
| Location | |
| Stomach | 24 (62) |
| Small bowel | 12 (30) |
| Large bowel | 2 (5) |
| Retroperitoneum | 1 (3) |
| Operative method Laparoscopy Open surgery | 10 (26) 29 (74) |
| Surgical margin status R0 R1 R2 | 31 (80) 3 (7.7) 5 (12.3) |
| Recurrence Local Distant metastasis Synchronus | 3 (23.1) 8 (61.5) 2 (15.4) |
| Survival status | |
| Alive | 29 (75) |
| Died from disease | 6 (15) |
| Died from other cause | 4 (10) |
Table 2: Pathological characteristics.
| Pathological characters | Number (%), (n=39, 100%) |
|---|---|
| Positive immunohistochemical staining | |
| CD 117 | 39 (100) |
| CD 34 | 33 (84.6) |
| SMA | 13 (33.3) |
| S100 | 3 (7.6) |
| Ki 67 | 4 (10.2) |
| Tumor size | |
| < 10 cm | 25 (64.1) |
| ≥ 10 cm | 14 (35.9) |
| Mitotic count | |
| < 5/50 HPF | 23 (59) |
| ≥ 5/50 HPF | 16 (41) |
| Cell type | |
| Spindle cell | 35 (89.7) |
| Epithelioid/ mixed | 4 (10.3) |
| Risk category | |
| Very low | 2 (5.1) |
| Low | 5 (12.8) |
| Intermediate | 10 (25.6) |
| High | 22 (56.4) |
Table 3: Characteristics of recurrent patients.
| Age (yr) | Gende r | Location of primary tumor | Size (cm) | Mitosi S | Margin status R0/R1/ R2 | Recurre nt location | PFS (mo) | os (mo) | End statu S |
|---|---|---|---|---|---|---|---|---|---|
| 39 | M | Small bowel | 21 | >10/5 OHPF | R1 | Local, liver | 4 | 7 | Dead |
| 60 | M | Small bowel | 8 | >10/1 OHPF | R2 rupture | Local, liver | 26 | 114 | Alive |
| 22 | M | Small bowel | 20 | 30/10 HPF | R2 rupture | liver | 4 | 23 | Dead |
| 78 | M | Stomach | 28 | >5/50 HPF | R0 | Local | 6 | 17 | Dead |
| 41 | F | Stomach | 8 | 35/50 HPF | R1 | liver | 4 | 54 | Alive |
| 71 | M | Stomach | 20.5 | 20/50 HPF | R0 | liver | 27 | 49 | Alive |
| 60 | M | Large bowel | 3.7 | 10/50 HPF | R0 | Local | 48 | 56 | Alive |
| 55 | F | Small bowel | 17 | >5/50 HPF | R1 | Liver | 75 | 87 | Alive |
Table 4: Univariate analysis of the prognostic factors.
| Factors | Number | Overall survival (Os) | Disease free survival (DFS) | ||
|---|---|---|---|---|---|
| n= 39 | 5-yr os (%) | P value | 5-yr DFS (%) | P-value | |
| Age (XL) <60 >60 | 18 21 | 81.4 83.7 | 0.881 | 80.4 85.7 | 0.775 |
| Sex | 0.25 | 0.3 | |||
| Female | 21 | 89.6 | 90.5 | ||
| Male | 18 | 74.5 | 75 | ||
| BMI (kg/m2) <20 >20 | 14 25 | 78.6 85.1 | 0.61 | 76.9 88 | 0.54 |
| Tumor size (cm) | 0.97 | 0.77 | |||
| <10 cm | 25 | 82.3 | 82.4 | ||
| >10 cm | 14 | 84.4 | 85.7 | ||
| Mitosis | 0.03* | 0.023* | |||
| <5/50 HPF | 23 | 94.7 | 95.7 | ||
| 5/50 HPF | 16 | 66.5 | 65.3 | ||
| Cell type Spindle Epithelioid/ mixed | 35 4 | 83.2 75 | 0.61 | 85.7 75 | 0.505 |
| Risk category | 0.49 | 0.14 | |||
| Very low/Low | 7 | 100 | 100 | ||
| Intermediate | 10 | 88.9 | 90 | ||
| High | 22 | 74.1 | 74.3 | ||
| Location | 0.015* | 0.041* | |||
| Stomach | 24 | 90.5 | 91 | ||
| Non-stomach | 15 | 69.8 | 70.7 | ||
| Surgical margin | 0.011* | 0.001* | |||
| R0 | 31 | 93.5 | 92.5 | ||
| R1 | 3 | 50 | 50 | ||
| R2/ rupture | 5 | 50 | 50 | ||
Discussion
This retrospective study, based on 39 patients with GIST, aimed to investigate the clinicopathological and prognostic characteristics of this disease. The results are comparable with those of previous studies in other populations [15-19]. The stomach was the most common site of tumor origin in this series. The distribution of sites was similar to that published in 1965 by Skandalakis [20]. Joensuu et al [21] pooled individual data from 3,067 patients and reported similar demographics of GISTs. They found that GIST patients had a slight female predominance with the median age of 60 years at the time of GIST detection. In addition, a review from Miettinen et al reported that the median age in the major series varied between 60–65 years [22].
Furthermore, positive immunohistochemistry staining for CD117 was 100%, which is supported by the previously study [23]. Radical tumor resection is the most important factor affecting patient prognosis. Furthermore, non-gastric disease location, higher mitotic rate and tumor metastasis or local invasion prior to treatment were revealed to be predictors of a poor prognosis [31-34]. There are no standard criteria for assessing the aggressive behavior and predicting the clinical prognosis of GISTs, although the NIH and AFIP criteria are widely use [7,8]. It is commonly accepted that all GISTs are considered to have malignant potential. Through univariate analysis, higher mitotic rate , non gastric location and incomplete resection or tumor rupture are associated with poor survival for GIST patients. Similarly, the British study [24] identified high mitotic index as an independent poor prognostic factor, and non-gastric disease location is associated with tumor recurrence, which is consistent with the results of previous studies [21]. Large tumor size is considered to be a prognostic factor in the NIH and AFIP risk classification criteria [16,18]. However, it is not an independent risk factor in the present study.
For resectable localized GISTs, radical surgery with clear margins without lymphadenectomy is the standard and forefront of curative treatment [25,33]. Radical tumor resection significantly improves survival and reduces risk of tumor recurrence [33]. Various surgical approaches, including open and laparoscopic surgery were performed in the present study. There was no significant difference in OS or DFS among these surgical strategies. Several studies comparing the effect of minimally invasive and open surgery in the treatment of GISTs were performed [25]. The results are generally accepted that minimally invasive surgery has similar or superior peri-operative outcomes [3,13] without compromising the oncological outcomes; it may also be safely applied for larger tumors or tumors located in unfavorable sites. Imatinib has an important role in the treatment of advanced GISTs and was used in the adjuvant setting to reduce the risk of recurrence and metastasis [26-34]. Recently, imatinib treatment as adjuvant therapy for patients with intermediate-to-high risk GIST are recommended [33,34]. However, there was no observed improvement in OS and DFS in the 10 patients with advance disease who received adjuvant imatinib therapy in our study. The possible reason is limited sample size. Imatinib was also administered to patients with incomplete resection and those with disease progression. Despite of the treatment, advanced disease will inevitably progress and associated with lower the OS and DFS rates.
With the median follow-up time of 46 months (1-139 months), the overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively ; which is higher than the results of DeMatteo et al, who reported 54% survival at 5 years in a group of 200 patients encountered during 16 years [13]. These results are similar to those published previously, which reported 5 year survival rates of 48% to 92.1% [9-14].
There were certain limitations to this study: The study design was retrospective; the selection of surgical approach and adjuvant therapy were not randomized, the use of imatinib was limited to 10 patients with a potential selection bias due approved indication for adjuvant treatment problem which is not covered by the universal health insurance system in Thailand. Only recurrence or metastasis disease that can be reached to therapy. Therefore, the benefit of using imatinib as adjuvant therapy cannot be evaluated based on this study.
Furthermore, the benefit of imatinib treatment for intermediate or high risk patients needs to be verified.
Conclusion
The findings of this retrospective review of 39 cases of GISTs at a single center showed, the overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively. R0 resection, mitotic figure and location of tumor were the significant prognostic factors.
The findings of this retrospective review of 39 cases of GISTs at a single center showed, the overall survival and disease-free survival at 5 years were 84.6% and 66.7%, respectively. R0 resection, mitotic figure and location of tumor were the significant prognostic factors.
Disclosure statement: All authors declare that they have no conflict of interest.
Ethical approval: Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine, vajira Hospital, Navamindradhiraj University. Protocol Number: ID 086/61.
Author contribution: The authors confirm contribution to the paper as follows: study conception and design: Putticha Keawmanee, Wisit Kasetsermwiriya, Suphakarn Techapongsatorn; data collection: Putticha Keawmanee; analysis and interpretation of results: Wisit Kasetsermwiriya, Putticha Keawmanee; draft manuscript preparation: Putticha Keawmanee, Wisit Kasetsermwiriya, Suphakarn Techapongsatorn, Nathan Sirivongs. All authors reviewed the results and approved the final version of the manuscript.
References
- Mazur MT and Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983; 7: 507-519.
- Goettsch WG, Bos SD, Breekveldt-Postma N, et al. Incidence of gastrointestinal stromal tumours is underestimated: Results of a nation-wide study. Eur J Cancer. 2005; 41: 2868-2872.
- Wang M, XU J, Zhang Y, Tu L, Qiu WQ, et al. Gastrointestinal stromal tumor: 15-years' experience in a single center. BMC Surg. 2014; 14: 93.
- Pisters PW, Blanke CD, von Mehren M, et al. A USA registry of gastrointestinal stromal tumor patients: Changes in practice over time and differences between community and academic practices. Ann Oncol. 2011; 22: 2523-2529.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23: 70-83.
- Yanagimoto Y, Takahashi T, Muguruma K, et al. Reappraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015; 18: 426-33.
- Goh BK, Chow PK, Yap WM, Kesavan SM, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified armed forces institute of pathology risk criteria. Ann Surg Oncol. 2008; 15: 2153-2163.
- Belfiori G, Sartelli M, Cardinali L, et al. Risk stratification systems for surgically treated localized primary gastrointestinal stromal tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC nomogramm, NIH-fletcher and AFIP-miettinen. Ann Ital Chir. 2015; 86: 219-227.
- Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer. 1978; 42: 1375-1384.
- Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982; 49: 177-187.
- McGrath PC, Neifeld JP, Lawrence WJ, Kay S, Horsley JS, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg. 1987; 206: 706-710.
- Ng EH, Pollock RE, Munsell MF, Atkinson EN. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992; 215: 68-77.
- Ronald P. DeMatteo, Jonathan J. Lewis, et al. Two Hundred Gastrointestinal Stromal Tumors Recurrence Patterns and Prognostic Factors for Survival. Ann Surg. 2000; 231: 51-58.
- Kasetsermviriya w, Nagai E, et al. Surgery of upper GI gastrointestinal stromal tumors:Our experience, Prognostic analysis. Hepato-gastroenterol. 2015; 62: 87-92.
- Tran T, Davila Ja and El-serag HB: The epidemiology of malignant gastrointestinal stromal tumors: An analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 100: 162-168, 2005.
- Tryggvason G, Gislason HG, et al. Gastrointestinal stromal tumors in Iceland, 1990-2003: The icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005; 117: 289293.
- Rubio J, Marcos-Gragera R, Ortiz MR, Miró J, et al. Population-based incidence and survival of gastrointestinal stromal tumours (GIST) in girona, spain. Eur J Cancer. 2007; 43: 144-148.
- Mucciarini C, Rossi G, Bertolini F, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007; 7: 230.
- Chiang NJ, Chen LT, Tsai CR and Chang JS. The epidemiology of gastrointestinal stromal tumors in taiwan, 1998-2008: A nation-wide cancer registry-based study. BMC Cancer. 2014; 14: 102.
- Skandalakis JE, Gray SW. Smooth muscle tumors of the alimentary tract. In: Ariel IM, ed. Progress in Clinical Cancer. New York: Grune & Stratton; 1965: 692-708.
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013; 382: 973-83.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013; 42: 399415.
- Wu TJ, Lee LY, Yeh CN, et al. Surgical treatment and prognostic analysis for gastrointestinal stromal tumors (GISTs) of the small intestine: before the era of imatinib mesylate. BMC Gastroenterol. 2006; 6: 29.
- Mrowiec S, Jabtonska B, Liszka L, et al. Prognostic factors for survival post surgery for patients with gastrointestinal stromal tumors. Eur Surg Res. 2012; 48: 3-9.
- Novitsky YW, Kercher KW, Sing RF and Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006; 243: 738-745, 745-747.
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007; 25: 1107-13.
- Wang C, Zheng B, Chen Y, et al. Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs. Chin J Cancer Res. 2013; 25: 63-70.
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017; 29: 281-293.
- Eisenberg BL, Trent JC. Adjuvant and neoadjuvant imatinib therapy: Current role in the management of gastrointestinal stromal tumors. Int J Cancer. 2011; 129: 2533-42.
- Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010; 97: 1854-9.
- Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007; 14: 2018-27.
- Liu Q, Li Y, Dong M, Kong F and Dong Q. Gastrointestinal bleeding is an independent risk factor for poor prognosis in GIST patients. Biomed Res Int . 2017: 7152406.
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25: iii21–iii26.
- Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016; 19: 3–14.