Japanese Journal of Gastroenterology Research
Review Article - Open Access, Volume 1
Effects of probiotics on inflammatory bowel disease: A systematic review
Gabriela Lopes1; Gyovanna Sorrentino dos Santos Campanari1; Sandra M Barbalho1,2,3*; Júlia Novaes Matias1; Vinícius Marinho Lima1; Adriano Cressoni Araújo1,2; Ricardo de Alvares Goulart2; Claudia Rucco P Detregiachi2; Jesselina F Santos Haber1; Antonelly Cassio Alves de Carvalho2; Luiz Henrique Alvarenga2; Thais de Oliveira Ullman2; Patrícia C Santos Bueno1; Élen Landgraf Guiguer1,2,3
1Department of Biochemistry and Pharmacology, School of Medicine, University of Marília (UNIMAR), Avenida Higino Muzzi Filho, 1001, Marília, São Paulo, Brazil.
2Postgraduate Program in Structural and Functional Interactions in Rehabilitation-UNIMAR – Marília-SP, Brazil.
3School of Food and Technology of Marilia (FATEC) – Marilia – SP, Brazil.
*Corresponding Author: Sandra Maria Barbalho
Department of Biochemistry and Pharmacology, School of Medicine, University of Marília, Av. Higino Muzzi Filho 1001, Marília 15525-902, SP, Brazil.
Tel: 55-14-99655-3190
Email: smbarbalho@gmail.com
Received : Jun 25, 2021
Accepted : Aug 04, 2021
Published : Aug 12, 2021
Archived : www.jjgastro.com
Copyright :© Barbalho SM (2021).
Abstract
Inflammatory Bowel Disease (IBD) is a chronic idiopathic inflammation of the gastrointestinal tract, characterized by periods of exacerbation and remission, which includes two primary forms: Ulcerative Colitis (UC) and Crohn's Disease (CD). IBD is an autoimmune disorder influenced by genetics, hereditary, environment, and infection. Alternative treatments with fewer side effects have been searched. Thus, the use of probiotics stands out due to several beneficial effects. For this reason, the objective of this systematic review is to evaluate the effects of the use of probiotics in patients diagnosed with IBD. This review included studies available in the MEDLINE databases - PubMed, EMBASE, and Cochrane, and the final selection included thirteen randomized clinical trials. The results showed that the oral use of probiotics and, mainly for a prolonged period, can help in the improvement of reducing symptoms related to IBD. In endoscopic analyzes, a reduction in intestinal inflammation was noted, in addition to a reduction in proinflammatory cytokines. Although there are positive effects, there is a need for further studies to define the best composition of probiotics and time of use since there are many variations in this treatment.
Keywords: probiotics; inflammatory bowel disease; crohn's disease; ulcerative colitis.
Citation: Lopes G, Campanari GSDS, Barbalho SM, Matias JN, Lima VM, et al. Effects of probiotics on inflammatory bowel disease: A systematic review . Japanese J Gastroenterol Res. 2021; 1(2): 1009.
Introduction
Inflammatory Bowel Disease (IBD) is a multifactorial condition related to a complex interaction between immunity, genetics, epigenetics, and even intestinal microbiota. Among IBD, Ulcerative Colitis (UC) and Crohn's Disease (CD) are the primary clinical forms that have some distinct points, such as location and injury. CD affects the terminal portion of the ileum and the colon, while the UC primarily affects the colon and rectum. They are both chronic diseases, with periods of crises and remissions, and may present abdominal pain, diarrhea with blood and mucus or constipation, weight loss, and even surgical approach is needed in more severe cases. Extraintestinal manifestations, such as fever, liver, lung, cardiovascular, and skin disorders may be present, showing a systemic and potentially serious involvement of this condition [1,2].
Although the pathogenic mechanisms of IBD are not yet completely elucidated, it is known that intestinal inflammation is related to an exacerbated response of the immune system due to a deregulation of several factors, including gut microbiota and genetic aspects. Recent studies indicate that interactions between the patient and his microbiota play a prominent role in IBD architecture, involving portions of the genome responsible for regulating microbial defense and intestinal inflammation. It was perceived that in the case of UC and CD, there is an important reduction in Lactobacillus and Bifidobacterium [1].
The human gut microbiota contains bacteria, fungi, viruses, and protozoa. More than 99% of the bacteria belong to Bacteroidetes, Firmicutes, Proteobacteria and Actinobacteria and the composition and number of these microorganism are associated with the maintenance of homeostasis. Modifications in the composition and function of microbiota is named dysbiosis, which is linked to several diseases, such as IBD. In this condition, there is augment of inflammatory biomarkers due to the decrease of bacteria with anti-inflammatory ability and the release of metabolites associated with flare episodes of IBD. Moreover, typical drugs used to treat IBD, such as mesalazine, diminishes fecal bacteria and the mucosal adherent bacteria if compared to control patients [3,4].
Even though there is no effective cure for IBD, there are some treatments for controlling the disease, aiming to achieve the most extended remission duration. The conventional treatments include chronic use of antibiotics, corticosteroids, immunosuppressants, and anti-Tumor Necrosis Factor (TNF). In general, these drugs are not always able to keep the patient in remission, and it may be necessary to combine classes of drugs whose side effects are extensive and worsen patients' quality of life. These effects may include hemopathies, diarrhea, vomiting, and thrombocytopenia. However, a strong relation between IBD and dysbiosis has been observed since it is common to find the gut microbiome significantly altered in several intestinal diseases. For these reasons, alternative and complementary treatments such as probiotics could help the patient recover [5-7].
Probiotics are characterized as living microorganisms that, when administered in adequate amounts, alter the intestinal biota. Studies have shown that dysbiosis and changes in bacterial metabolic pathways are essential factors for the onset of the first symptoms and disease progression. When treated with probiotics, they show significant improvement, decreasing intestinal inflammation, pain, swelling, and quality of life, since they help restore the mucosa and promote the anti-inflammatory effect. It is also important to notice that the cost of probiotics and their potential side effects are much lower than conventional treatment [8,9].
Taking into account that the current treatments available are not always effective in controlling symptoms and in sustaining remission, added to the many side effects and high cost, enforced by the close relation of the intestinal microbiota and pathophysiology of IBD, this study aims to review the effects of the use of the probiotic in patients with IBD.
Methods
Focused question
This review aimed to answer the following question: Can Probiotics exert beneficial effects on Inflammatory Bowel Disease
Language
The inclusion criteria were only studies in English.
Databases
This review included studies found in MEDLINE–PubMed (National Library of Medicine, National Institutes of Health), EMBASE, and Cochrane databases. The Mesh terms that were used included Probiotics or Lactobacillus or Bifidobacterium or Enterococcus or Bacillus mesentericus or Clostridium butyricum or Streptococcus and Inflammatory Bowel Disease or Ulcerative Colitis or Crohn's Disease which helped to select studies related to the use of Probiotics and its effects on Inflammatory Bowel Disease treatment. The authors have followed the PRISMA (Preferred Reporting Items for a Systematic Review and Meta-Analysis) guidelines.
Study selection
This study contains only studies that described the use of probiotics as adjuvant therapy for IBD, associated or not with symptomatic drugs or standard treatment.
The inclusion criteria for this search were Randomized Clinical Trials (RCTs). Other sources were consulted to build the introduction and discussion but were not included in Table 1 and Table 2.
The exclusion criteria were reviews, studies not in English, editorials, case reports, and poster presentations.
Eligible criteria
The eligible criteria for this review followed the PICO (Population, Intervention, Comparison, and Outcomes) format for RCT. The outcomes were a reduction in IBD scores, reduction of proinflammatory biomarkers, increased helpful bacteria such as Lactobacillus and Bifidobacterium, and improvement in the quality of life. Only full studies published in the consulted databases were selected.
Data extraction
Two independent judges performed the search for the studies to identify the RCT in the databases. The abstracts of the papers were evaluated, and only full-text studies were retrieved to support the decision-making process. Disagreements between the judges were evaluated and decided by two other reviewers.
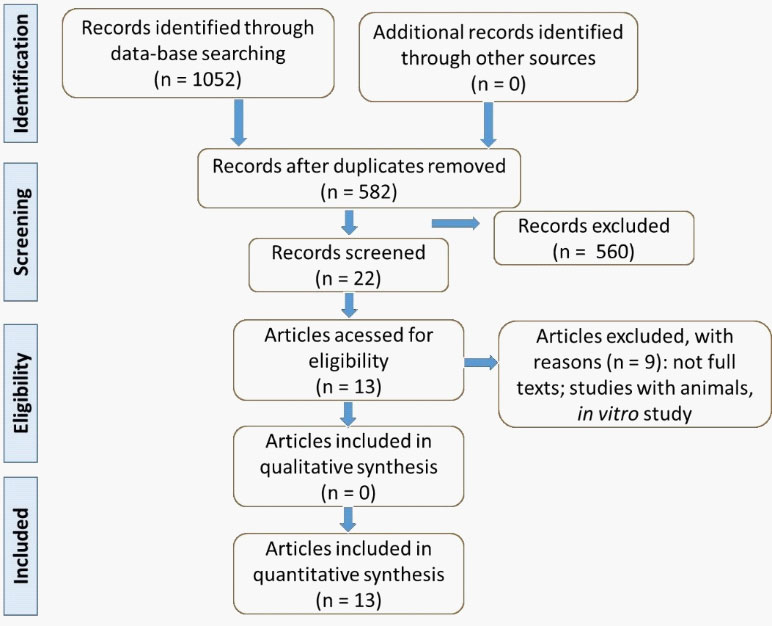
The selected articles included studies from 2015 to 2020 and, after identifying the articles available, only other studies presented in the flow chart (Figure 1) filled the objectives of this review.
Quality assessment
The evaluation of the risk of bias (randomization, selection, detection, and reporting bias of each RCT) followed the Cochrane Handbook for Systematic Reviews of Interventions.
Results
The selection of the studies is presented in Figure 1, and studies are found in Table 1 and Table 2. Thirteen studies were selected to compose this review; all of them were randomized clinical trials. Altogether, 1,198 individuals were registered in the selected studies, aged 19-76 years old (531 men; 504 women).
In the article by Bjarnason et al. [1], there was no specification of the exact number of men and women included in the study. In the article by Bin et al. [10], there was a gender specification of the participants only in the group diagnosed with IBD, with this specification not occurring in the healthy group. Thence, we did not include these participants in the total described above.
Discussion
Pathophysiological mechanisms of IBD
Inflammatory Bowel Disease is idiopathic chronic inflammation of the gastrointestinal tract. UC is an inflammation of the mucosa and submucosa of the colon and rectum, while CD is a transmural inflammation that can affect the whole gastrointestinal tract, mainly reaching the terminal ileum, colon, and perianal region. The etiology and mechanisms behind these diseases have not yet been totally clarified. However, authors have been considered both an autoimmune disorder influenced by hereditary, genetics (mutations in NOD2, TLR, OCTN1/2, ATG16L1, and IRGM genes), environment (high sugar, high fat, pollution, smoking, drugs, sleep, stress, and Western-type diet) and infection [2,11-13].
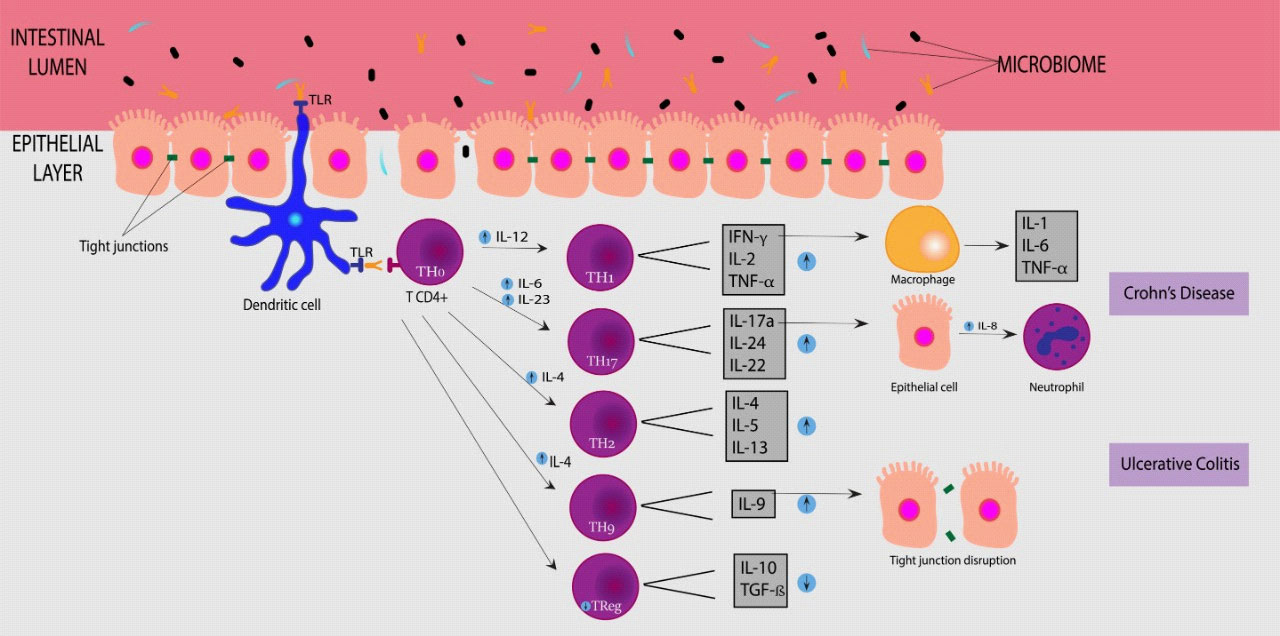
Alteration in innate and acquired immunity of the intestinal mucosa are the main mechanisms related to the disease. Macrophages show high secretion of TNF-α, IL-1β, IL-12, high expression of CD14 receptor, and goblet cell secretion inhibition, destroying epithelial cell barrier. Neutrophils present over-recruitment and activation, leading to severe injury of the mucosa. Dendritic cells exhibit over-expression of TLR2 and TLR4 and high production of IL-12 and IL-6, unbalancing CD4+ T cell differentiation. Th1 cells are differentiated by IL-12 and IL-27 and are characterized by the secretion of IFN-γ, TNF-α, and IL-2. On the other hand, Th2 differentiation is caused by IL-4 stimulation, and these cells secrete IL-4, IL-5, and IL-13. The increase in IFN-γ induces macrophages to secret TNF, the main cytokine involved in inflammation of IBD, promoting the transcription of other proinflammatory cytokines, up-regulation of adhesion molecules in the endothelium, and activation of phagocytic activity of macrophages. IL-17, IL-21 characterize Th17 and IL-22 secretion and are stimulated by IL-23, IL-6, and TGF-β and can induce IL-8 production by epithelial cell (neutrophil recruitment), while IL-21 leads to the secretion of IL-17A and IFN-γ. The Th9 cells also participate in IBD pathology, characterized by the production of IL-9 and induced by IL-4 and TGF-β, causing a block in the proliferation of intestinal epithelial cells and down-regulating the expression of tight-junction proteins, including claudin and occludin. Lastly, Treg is a CD4 T-cell suppressor by secreting anti-inflammatory cytokines, such as IL-10 and TGF-β, inhibiting Dendritic cells and macrophages [11,13-15].
Recent studies show that UC and CD have different cell activation: a Th1 response drives CD, and UC is driven by non-conventional Th2 and Th9 responses. Th17 are found in both forms of IBD. Tregs are found to be diminished in the blood of IBD patients but increased in lamina propria, although T-cells of IBD patients may be resistant or less responsive to Tregs suppression. Lastly, B-cells show an increase in immunoglobulin G (IgG) production in UC and CD, directed to commensal bacteria. These alterations in immunity lead to an abnormal reaction to commensal bacteria, changing the intestinal microflora and causing dysbiosis, a reduction in the number of commensal bacteria, and an increase of pathogenic microorganisms [2,11,14,16,17]. These mechanisms of IBD are shown in Figure 2.
General aspects of probiotics
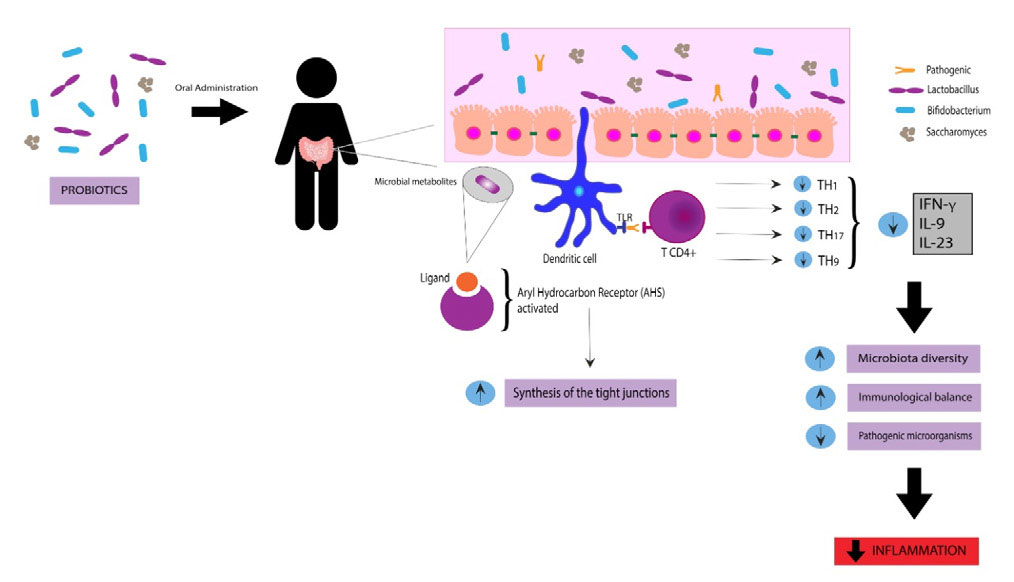
In accordance with the World Health Organization, probiotics are defined by live microorganisms that, if consumed in suitable proportions, provide health benefits to the host. Probiotics provide a protective effect on the gastrointestinal tract microbiota by colonization or transient activity in some species. They consist of most bacteria or fungus similar to the commensal microorganisms found naturally in the human gastrointestinal tract and can be administered in many different forms, including combined forms, supplementation, or functional foods. The most studied species are Lactobacillus, Bifidobacterium, and Saccharomyces. The exact mechanisms of probiotics are unknown, but it seems to increase microbiota diversity, maintain immunologic equilibrium, and reduce colonization by pathogenic microorganisms [18-20]. Figure 3 show some aspects of the mechanism of action of probiotics.
More than 25 diseases are associated with microbiota alteration, including autoimmune diseases, emotional disorders, obesity, acute infectious diarrhea, hepatic encephalopathy, necrotizing enterocolitis, IBD, and Irritable Bowel Syndrome (IBS), which could benefit from the use of probiotics. The use of probiotics is safe in every age, more cautiously in immunosuppressed patients [19-21].
On the other hand, the definition of prebiotics includes selectively fermented compounds that can lead to specific modifications in the composition and/or activities of the gastrointestinal microbiota, resulting in benefits upon host health. Most of them are dietary fibers fermented by intestinal microbiota, which stimulates intestinal bacteria's growth and/or activity. Examples of these ingredients are mucin, fermented by Faecalibacterium prausnitzii and Akkermensia muciniphila, and oligofructose, fermented by Bifidobacterium, Bacteroides, Faecalibacterium, Lactobacillus, and Roseburia in vitro. The response to these compositions varies among the population, mostly because of the variability of the microbiome and the dosage of prebiotics used [18,22,23].
Lastly, Symbiotic is combined product containing probiotics and prebiotics in one synergistic form, capable of decrease intestinal inflammation and increase the epithelium barrier activity. The addition of prebiotics to probiotics treatment improves the probiotic proliferation and increases local microbiota proliferation, such as Bifidobacterium animalis in rats [22,24].
Use of probiotics on IBD
Since traditional treatment for IBD includes aminosalicylates, corticosteroids, anti-TNF (such as infliximab, adalimumab, certolizumab), anti-interleukins (ustekinumab), anti-integrins (vedolizumab), janus kinase inhibitors, leukocyte trafficking/migrating inhibitors and other drugs that can be related to side effects (such as diarrhea, lymphopenia, and opportunistic infections), there is a need for other adjuvant therapies. However, authors have shown a relationship between the intestinal microbiota and IBD since dysbiosis can be associated with inflammatory processes, immunological imbalance, oxidative stress, and an increase in inflammatory mediators. MicroRNAs (miRNAs) acting on pathways of the immune response and gene expression were also noted. These miRNAs are influenced by the intestinal microbiota, which demonstrates that there is inadequate production of miRNAs and development of IBD if there is dysbiosis. The use of probiotics started to draw attention due to their ability to improve the epithelial barrier's function, homeostasis, and immunological system modulation. The main bacterial strains used as treatment are Lactobacillus and Bifidobacterium, both of which are useful for treating UC. The positive effects of the strains used in probiotics depend on their antioxidant capacity and the modulation of miRNAs since they will act on homeostasis compromised by intestinal inflammation [25-28].
Besides, it can be noted that there are several immunological mechanisms involved in the disease's pathogenesis, so there are several etiologies involved in IBD. Intestinal dysbiosis can cause a chronic inflammatory state, with MALT activation (Mucosal Associated Lymphoid Tissue), leading to IBD development. The high levels of TNF-α, IFN-y, and IL-23 proved to be able to stimulate the breakdown of the epithelial barrier, mainly by compromising the expression of the tight junction's protein ZO-1. However, after the administration of specific probiotics, this compromise improves through several mechanisms, one of which is the synthesis of tight junction proteins through the activation of the aryl hydrocarbon receptor, leading to the increase in the synthesis of ZO-1 (zonula occludens-1), improving the functionality of the epithelial barrier, as shown in Figure 3. Although there are several mechanisms involved in IBD's pathophysiology, the use of probiotics has beneficial effects due to different mechanisms of action [29,30]. In Table 1 we show the studies included in this review.
Yilmaz et al. [9] (Table 1) included UC and CD patients that received Kefir, a mixture of Lactobacillus. Patients were asked to fill out the symptoms diary with questionnaires of bowel habits, like abdominal pain, stool consistency, and feeling good. All patients underwent hemoglobin (CRP, and erythrocyte sedimentation rate evaluations, and the clinical activity index was calculated before and after the treatment. Stool analysis was performed before and after treatment to measure the amount of Lactobacillus. The results indicated that regular use of Kefir could improve symptoms and short-term quality of life in patients with CD and positively affect biochemical parameters. In addition, in two weeks of treatment, there was an improvement in bloating symptoms, abdominal pain, and quality of life. Better results were noted in patients with CD than in patients with UC in terms of improved quality of life and worse results in abdominal pain in patients with CD in the last two weeks of treatment. A possible bias of this RCT is that patients in the control group did not receive a placebo. Beiond that, the number of participants was small, and the study was performed for a short period of time.
Fan et al. [31] carried out a study with IBD patients that receive Pentasa® (mesalazine) or Pentasa® plus probiotics. The two groups' activity scores were calculated. After treatment, CDAI and UCAI were much lower when compared to the group without probiotics. Although the study concluded that the combination of probiotics and Pentasa® could improve microflora composition in patients with IBD and reduce the level of inflammatory cytokines, the research was carried out with a very small sample of patients, which interferes with the significance of the data.
The research performed by Bjarnason et al. [1] (Table 1) investigated the use of Symprove® probiotics (Enterococcus faecium, Lactobacillus plantarum, Streptococcus thermophilus, Bifidobacterium lactis, Lactobacillus acidophilus, Bifido-long bacteria, and fructooligosaccharide) in UC and CD patients and concluded that this multi-strain probiotic is related to reduced intestinal inflammation in subjects with UC, but not in CD, and is well-tolerated. There were no side effects reported in the survey. The probiotic or placebo administration was made by the patient himself, which may have cooperated to not adherence to the treatment or used the medication without following the fasting prescription.
Kamarli et al. [32] carried out a study with UC patients treated with probiotics (Table 1). Most patients in the study needed to be treated with mesalazine or a combination of mesalazine and azathioprine, with no significant difference compared to the placebo and probiotic groups. At the end of the study, most subjects (55.6%) had gone into remission. According to UCEIS, the results were similar: patients using the probiotic obtained significant improvement, with remission of a large part of the group, compared with the placebo group. There are limitations in the study, such as the small sample. In addition, no analyzes of inflammatory markers were made, which could cooperate in analyzing disease activity in patients. Patients were treated for a short period, making it impossible to analyze the probiotic's long-term effects.
The research conducted by Bamba et al. [33] (Table 1) investigated the effects of a fermented vegetable (Pediococcus pentosaceus) in UC patients. A large dropout of patients was observed for personal reasons. Patients tended to continuously exhibit higher levels of acetic acid, propionic acid, and n‑butyric acid, while their levels of lactic acid tended to decrease following consumption of the fermented vegetable beverage. The amount of Bifidobacterium tended to be lower in patients using the probiotic. Levels of lactic acid were also lower after consuming the probiotic. The authors concluded that the 8-w consumption of the fermented vegetable beverage by UC patients marginally improved loose stool symptoms but did not affect the UC disease activity but improved the intestinal environment. In this trial, it is possible to notice some biases, such as having a very small sample and suffering a great dropout from the participating patients. Although the selected patients had active UC, the treatment was performed for a short period, preventing long-term analysis, such as analyzing the disease remission process and possible recurrences.
The study of Matsuoka et al. [34] (Table 1) was performed with UC patients that received fermented milk. In this study, there is an insufficient number of bacteria for treatment, which can interfere with probiotic results. Another bias is that the patients were in remission, and the treatment was carried out for a short period of time, which may have interfered with the results. The study was discontinued due to an ethical issue as it was not considered useful in keeping patients in remission.
Bin et al. [10] (Table 1) performed a study with UC patients and patients with a food allergy, and UC that received immunotherapy and probiotic; only probiotic; specific immunotherapeutic only; and placebo for one year. The cytokines, such as IL-4, IL-13, IFN-y, and TNF-α, were higher in patients with food allergy. However, when performing the culture-specific antigen test, the CD4+ T cells of patients with food allergy and UC produced more IL-4 than the other groups. This result indicated a specific Th2 immune response to body antigens in patients with food allergies and UC. The authors concluded that the treatment with specific immunotherapy and probiotics could markedly improve the immunity, clinical symptoms, and reduction of using UC-control medicines of food allergy and UC patients.
Palumbo et al. [35] (Table 1) performed a trial with UC patients and every six months, patients were analyzed using the Modified Mayo Disease Activity Index (MMDAI) (the study excluded patients who used glucocorticoids, patients with renal failure, pregnant women and lactating women). There was an improvement in stool frequency, with a more significant reduction in frequency at 6 and 24 months. At endoscopy, there was a significant improvement in the intestinal mucosa. However, the study was limited due to having a small sample of patients.
The study of Tamaki et al. [36] (Table 1) enrolled 5patients with mild to moderate UC treated with placebo or Bifidobacterium longum and drugs such as 5-ASA, prednisolone, azathioprine, and 6-mercaptopurine, in constant dose during the treatment. The study concluded that BB536, in addition to standard treatment, improves clinical symptoms and endoscopy findings in UC patients, mainly rectal bleeding and mucosal findings. Despite that, the study was performed with a small sample, and the participants were receiving different standard treatments concomitant to BB536, which may interfere in the results.
Yasueda et al. [37] (Table 1) performed a trial with CD patients who underwent a total proctocolectomy with ileal pouch-anal anastomosis treated with a probiotic composed of Clostridium butyricum MIYAIRI. There was also a difference in the level of Bifidobacterium and Bacteroides, being greater in the placebo group than in the probiotic group after treatment. In blood analyzes, there was no difference between groups regarding total serum proteins; however, there was a decrease in CRP after treatment. It was concluded that probiotic therapy using CBM might be a useful complementary therapy with minimal side effects for preventing pouchitis in patients with UC who have undergone IPAA. However, this investigation was carried out with a very small sample.
Fedorak et al. [38] (Table 1) investigated the use of VSL probiotics (Lactobacillus paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp bulgaricus, Bifidobacterium longum, B. breve, and B. infantis and Streptococcus salivarius subsp thermophiles) in patients with CD after two or three weeks after resection surgery The rate of severe recurrence was not statistically different among individuals on day 90; however, it was observed that patients who received probiotics from the beginning of the research until day 365 obtained a reduction in the recurrence of endoscopic lesions and reduced the levels of inflammatory cytokines compared to placebo, but those who started the probiotic later did not experience a significant reduction in cytokines on day 365. The authors concluded that the early treatment with the probiotic promoted beneficial effects than the late treatment in the post-surgical period, in addition to a decrease in the lesions (observed in endoscopy) and lower levels of proinflammatory cytokines.
Shadnoush et al. [39] (Table 1) conducted a trial performed with UC and CD patients in remission that received yogurt with probiotics (Lactobacillus acidophilus La-5 and Bifidobacterium BB-12). Improvement of intestinal function was observed due to the increase in helpful bacteria. The patients were asymptomatic, which does not allow an analysis of the clinical improvement of patients who were not in remission. Finally, the treatment was carried out for only eight weeks, which allows analyzing only the probiotic's short-term effects.
Yoshimatsu et al. [40] (Table 1) performed a study with UC patients in remission that received the probiotic Bio-Three (10mg of Streptococcus faecalis T-110, Clostridium butyricum TO-A, Bacillus mesentericus) or placebo and found that probiotics may be useful for maintaining clinical remission in patients with quiescent UC, especially those who belong to cluster I on fecal bacterial analysis, in addition to being effective in preventing relapse rates. An important bias of this study is a very small sample.
It is noted that the genus of bacteria most used was Lactobacillus, with 7 studies that used these bacteria. Among them, the most chosen were L. acidophilus (n = 6), L. plantarum (n = 3); L. paracasei (n = 1); L. bulgaricus (n = 1); and L. salivarius (n = 1). Only one of the studies that did not specify which types of Lactobacillus were used. In these groups, six concluded that the use of this type of probiotic improves the patient's quality of life, with the improvement of symptoms, reduction of intestinal inflammation, especially in UC. They also observed that this type of probiotic association with conventional treatment drugs, such as mesalazine, leads to more significant results, with improved stool frequency and more effective clinical response. However, 2 found significant differences between the placebo group and the group treated with probiotics when used for a prolonged period of treatment, with reduced inflammation of the intestinal mucosa and decreased recurrence rate. Three studies found no significant improvement. Bifidobacterium was also widely used, with 6 studies using this bacterium. Among them, the most used were B. longum (n =3 studies). However, B. lactis (n = 1); B bifidum (n = 1); B. infantis (n = 1), and B. breve (n = 2) were also used. One study did not specify which Bifidobacterium was evaluated. The results were very similar to Lactobacillus, with a clinical and endoscopic improvement of the patients. Besides, three studies noticed a more intense improvement, with a reduction of recurrences after prolonged use. In only one study, there was no significant improvement in patients with the use of the probiotic.
Other types of bacteria were also used in several studies, such as Clostridium (n = 3), Bificus (n = 1), Streptococcus (n = 3), Enterococcus (n = 2), and Pediococcus (n = 1). In these studies, the clinical and endoscopic improvement of patients was also observed. However, it is important to highlight that many studies have associated bacteria with composing the administered probiotic, which can interfere in the evaluation but guarantee an improvement of these patients by the association of the effects.
Regarding the side effects, Vahabnezhad et al. [41] reported a case of bacteremia in a 17-year-old patient diagnosed with CD with a recent diagnosis of Clostridium difficile infection and adenovirus. In this case, even though there is a possibility of contamination of Lactobacillus from the microbiota, analyzes showed that the contamination probably occurred due to the use of Lactobacillus that the patient received from his parents. In this case, previous infection by C. difficile and adenovirus, associated with CD, may have altered the colonic mucosa, facilitating the passage of Lactobacillus to the systemic circulation. Additionally, the patient was treated with glucocorticoids and infliximab, with consequent immunosuppression. All of these factors may have contributed to an increased susceptibility to infection. In the trials that were included in our review, when reported, adverse effects were mild and included nausea and vomiting [31], bloating, stress and odor change [34], dry cough [36] and infection in a post-surgical wound ([38], who evaluated the use of probiotics in patients with CD who underwent surgery.). These studies concluded that the use of probiotics is safe and well tolerated.
Table 1: Descriptive table of the selected randomized clinical trials.
| Reference | Model | Probiotics | Interventions | Outcomes |
|---|---|---|---|---|
| Yilmaz et al., [9] | Single-center, prospective, open-label, randomized, controlled trial, performed with 45 patients (23 men; 22 women) with CD (n=20) and UC (n=25), 33-42 y. | Kefir: Lactobacillus bactéria. | Patients with IBD were classified into two groups: treatment (n=25) and control (n=20). A 400 mL/day kefir was administered to the patients/4xdorally. The CDAI and Truelove-Witts scoring systems were used for the disease assessment scores. | There was an improvement in swelling, and in the well-being score in patients with CD, and an improvement in abdominal pain in patients with UC. No patient noticed worsening of symptoms or side effects |
| Fan et al., [31] | Single-center, prospective, randomized, controlled trial, performed with 40 patients (20 men; 20 women) with CD (n=9) and UC (n=31), 31-49y | Bifico capsule | Patients were divided to received 1-2 Pentasa® (mesalazine) tablets orally once and 3xd, or to received 2 probiotics tables orally once and 3xd in addition to the Pentasa®/40d. Patients were evaluated using the CDAI and UCAI scores. | There was significant decrease in intestinal bacteria in both groups, with an increase in Bifidobacteria and Lactobacilli in the group using probiotics. Lower levels of fecal lactoferrin, serum 1-antitrypsin, and β2-microglobulin in the probiotic group was observed. After treatment, there was a decrease in inflammatory markers in treated groups. The CD and UC activity index was lower in the group treated with probiotics. |
| Bjarnason et al., [1] | Single-center, prospective, double-blind, randomized, placebo-controlled trial, performed with 142 UC patients (n=81) and CD patients (n=61), 26-61y | Symprove®: Lactobacillus plantarum, Lactobacillus acidophilus and Enterococcus faecium. | Patients received 4w of treatment with the probiotic (1 ml/kg/day) or placebo orally each morning on a fasting stomach. Foods and fluids were allowed 20 min later. The primary efficacy measure was the difference in change in the IBD QOL between probiotic vs. placebo at week 4. Secondary outcome measures included the change in laboratory findings, including FCAL. | There were no significant differences in IBD-QOL scores between groups. FCAL levels were significantly reduced in the UC patients receiving the probiotics as opposed to placebo. |
| Kamarli et al., [32] | Multi-center, prospective, double-blind, placebo-controlled, randomized trial, with 36 patients (19 men; 17 women) 28-58y, diagnosed with UC. | Enterococcus faecium, Lactobacillus plantarum, Streptococcus thermophilus, Bifidobacterium lactis, Lactobacillus acidophilus, Bifido-long bacteria, and fructooligosaccharide. | Placebo (n=18) and the probiotic (n=18) received chewable tablets orally after breakfast and dinner/8w. The treated group received (225 mg/tablet). UCEIS and Truelove-Witts Clinical Activity Index were applied at the beginning of the study and at the end of 8 weeks. | Treated group showed significant decrease in the CRP and sedimentation values. In both groups, a statistically significant improvement was observed in the clinical and endoscopic activity levels at the end of the treatment. When the groups were compared with each other, improvement in the clinical activity was significantly greater in the probiotic group. |
| Bamba et al., [33] | Single-center, prospective, open-label, randomized, controlled trial, performed with 11 active UC patients diagnosed (5 men; 6 women), 36-60y, divided into 2 groups. | Pediococcus pentosaceus | Patients were divided into the group that received the fermented vegetable beverage/8w(n=6) or the subjects were followed up for 8w following enrollment and then consumed the beverage over the ensuing 8w (n=5). The patients were evaluated by the clinical symptoms and gastrointestinal symptoms, | There was no significant change in endoscopic severity before and after treatment. However, there was a significant improvement in the evaluation of gastrointestinal symptoms and stools |
| Matsuoka et al., [34] | Multi-center, prospective, double-blind study, placebo-controlled, randomized trial, performed with 195 patients (100 men; 95 women) 20-70y, diagnosed with UC. | Yakult: - Bifidobacterium breve and Lactobacillus acidophilus | The patients were randomly divided into 2 groups: one received 100 mL of fermented milk (Yakult) orally/d (n=98). The other received 100 mL of placebo (n=97) for 46 d. | Relapse-free survival was not significantly different between the BFM and placebo groups, nor was the incidence of relapse. The study was discontinued for lack of efficacy. An analysis of fecal samples from a subgroup of patients revealed a significant decrease in Bifidobacterium species before relapse, regardless of the treatment group. |
| Palumbo et al., [35] | Single-center, prospective, open-label, randomized, controlled trial, with 60 UC patients (41 men; 19 women) divided into the groups that received probiotic or not. | Lactobacillus salivarius, Lactobacillus acidophilus and Bifidobacterium Bifidus strain BGN4 | Group A (n=30) received 1200 mg of oral mesalazine 1xd; group B (n=30) treated with 1200mg of oral mesalazine/d and a double administration of a probiotic. The treatment was carried out for 2y. The patients were evaluated every 6 m with MMDAI. | There was clinical improvement for patients using probiotics. There was an improvement in the frequency of stools. After 6m there was endoscopic improvement in the aspect of the intestinal mucosa, and reduction in rectal bleeding compared to the group without probiotics. Hb levels were maintained |
| Bin et al., [10] | Single-center, prospective, double-blind, randomized, controlled trial, performed with 152 UC patients (65 men; 87 women), 30-42y (80 patients with food allergy and UC, 72 only with UC), and 20 controls. | Clostridium butyricum | First, 172 patients (80 patients with food allergy and UC, 72 only with UC, and 20 healthy patients) underwent blood collection to evaluate IgE levels and underwent Skin Prick Tests with common allergens. The patients with UC and food allergy were equally divided into 4 groups, to be treated with specific immunotherapy and/or probiotic 420mg or placebo for 12m. | UC and food allergy group treated with probiotic showed UC symptom improvement; the specific immunotherapy and probiotic group showed marked improvement in UC symptoms. The combination with immunotherapy and probiotic significantly reduced the medication scores. IgE was significantly higher in food allergy and UC patients |
| Tamaki et al., [36] | Multicenter, prospective, double-blinded, placebo-controlled, randomized trial performed with 56 UC patients (27 men; 29 women), 30-58y. | Bifidobacterium longum | The participants received either probiotic (n=28) or placebo (n=28) 3x/day for 8 weeks, concomitant to standard treatment. UCDAI and EI between baseline and at week 8 of treatment were evaluated. | There was a significant decrease in UCDAI and EI at the end of the treatment in the probiotic group, which was not found in the placebo group. 63% of probiotic patients showed remission. |
| Yasueda et al., [37] | Single-center, prospective, double-blind, placebo-controlled, randomized trial, performed with 17 patients (9 men; 8 women), 34-47y diagnosed with CD. | Clostridium butyricum MIYAIRI | Patients who underwent total proctocolectomy with IPAA were randomly divided. One group (n = 9) received the probiotic; the other group (n = 8) received a placebo. 9 probiotic or placebo tablets were administered/1x/d. Laboratory evaluation, fecal microbiota, pouchitis development, and mPDAI score were analyzed. | One subject in the probiotic group and four subjects in the placebo group developed pouchitis. No side effects occurred in both groups. The levels of the Clostridium coccoides increased after therapy in the placebo group. The proportion of the Enterococcus group in both groups tended to decrease after therapy. |
| Fedorak et al., [38] | Multi-center, prospective, double-blind, randomized, placebo-controlled trial, performed with 120 CD patients (62 men; 58 women), 25-50y | VSL: Lactobacillus paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp bulgaricus, Bifidobacterium longum, B. breve, and B. infantis and Streptococcus salivarius subsp thermophiles. | One group received probiotic orally (n=59), another one received placebo (n=60), 2-3 weeks after resection surgery, 1xd. They were reevaluated after 30 and 90 d, using the CDAI and IBDQ. On the last day, they performed a colonoscopy to evaluate the UC recurrence Patients with mild or no recurrence at 90 days were reevaluated on days 180, 270 and 365. | At day 90, the proportion of patients with severe endoscopic lesions did not differ significantly between VSL (9.3%) and placebo (15.7%). Aggregate rates of severe recurrence (on days 90 and 365) were not statistically different in the early and late VSL group. Patients receiving VSL had reduced mucosal inflammatory cytokine levels compared with placebo at day 90. CDAII bowel disease quality of life scores were similar in the 2 groups. |
| Shadnoush et al., [39] | Single-center, prospective, double-blind, placebo-controlled, randomized trial with 260 patients (132 men; 128 women) 27-40y, diagnosed with UC (n=198) or CD (n= 22) in remission or healthy patients (n=95). | Lactobacillus acidophilus La-5 and Bifidobacterium BB-12 | Group A (n= 105):IBD patients that received 250g of yogurt with probiotic orally/d; group B (n=105), with IBD patients that received 250g of placebo and a control group of healthy individuals who received 250g of yogurt with probiotics/d. Stool samples were collected before and after the intervention. | The mean numbers of Lactobacillus, Bifidobacterium, and Bacteroides in group A were significantly increased compared to B. There were also significant differences in the mean numbers of either of three bacteria between group A and the healthy controls; however, these differences between two groups were observed both at baseline and the end of the intervention. |
| Yoshimatsu et al., [40] | Single-center, prospective, double-blind, placebo-controlled, randomized trial, performed with 46 patients (28 men; 18 women) 27-57y, diagnosed with UC. | Bio-Three: Streptococcus faecalis T-110, Clostridium butyricum TO-A, Bacillus mesentericus. | Patients in remission from UC were randomly divided into the group that received 9 pills (10mg of probiotics) orally/d (n=23) and the group that received placebo tablets (n=23), in addition to ongoing medications. Clinical symptoms were analyzed monthly. Stool samples were analyzed using the cluster. | The relapse rates in the Bio-Three and placebo groups were respectively 0.0% vs 17.4% at the third month, 8.7% vs 26.1% at the sixth month, and 21.7% vs 34.8% at the ninth month. In the twelfth, the remission rate was 69.5% in the Bio-Three group and 56.6% in the placebo. On cluster analysis of fecal flora, 7 patients belonged to cluster Ⅰ, 32 to cluster Ⅱ, and 7 to cluster Ⅲ. |
BFM: Bifidobacterium Breve Strain Yakult; CBM: Clostridium Butyricum MIYAIRI; CD: Crohn's Disease; CDAI: Crohn's Disease Activity Index; CRP: C-Reactive Protein; EI: Endoscopic Index; FC: Fecal Calprotectin; FCAL: Faecal Calprotectin; Hs‐CRP: High Sensitivity C‐Reactive Protein; IBD: Inflammatory Bowel Disease; IBDQ: Inflammatory Bowel Disease Questionnaire; Ige: Immunoglobulin E; IPAA: Ileal Pouch-Anal Anastomosis; MMDAI: Modified Mayo Disease Activity Index; MPDAI: Modified Pouchitis Disease Activity Index; QOL: Quality Of Life Questionnaire Results; TNF‐Α: Tumor Necrosis Factor Alpha; UC: Ulcerative Colitis; UCAI: Ulcerative Colitis Activity Index; UCDAI: Ulcerative Colitis Disease Activity Index; UCEIS: Ulcerative Colitis Endoscopic Index Of Severity; VSL: Visbiome Probiotic.
Table 2: Descriptive table of the biases of the included randomized clinical trials.
| Study | Question focus | Appropriate randomization | Allocation blinding | Double-blind | Losses (<20%) | Prognostics or demographic characteristics | Outcomes | Sample calculation | Adequate follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Yilmaz et al, [9] | Yes | Yes | No | No | Yes | Yes | Yes | NR | Yes |
| Fan et al., [31] | Yes | Yes | NR | NR | Yes | Yes | Yes | NR | No |
| Bjarnason et al., [1] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NO | Yes |
| Kamarli et al., [32] | Yes | Yes | Yes | NR | Yes | Yes | Yes | NR | Yes |
| Bamba et al., [33] | Yes | Yes | N | No | No | Yes | Yes | NR | Yes |
| Matsuoka et al., [34] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Palumbo et al., [35] | Yes | NR | NO | NO | NR | NO | Yes | NO | Yes |
| Bin et al., [10] | Yes | NR | Yes | Yes | NR | Yes | Yes | NO | Yes |
| Tamaki et al., [36] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NO | Yes |
| Yasueda et al., [37] | Yes | No | Yes | No | Yes | Yes | Yes | NO | Yes |
| Fedorak et al., [38] | Yes | Yes | Yes | Yes | NO | Yes | NO | Yes | Yes |
| Shadnoush et al., [39] | Yes | NR | Yes | Yes | Yes | Yes | Yes | NR | Yes |
| Yoshimatsu et al., [40] | Yes | Yes | Yes | Yes | NR | Yes | Yes | NO | Yes |
NR: not reported.
References
- Bjarnason I, Sission G, Hayee B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn's disease. Inflammopharmacology. 2019; 27: 465-473.
- Abraham BP, Ahmed T, Ali T. Inflammatory Bowel Disease: Pathophysiology and Current Therapeutic Approaches. Handb Exp Pharmacol. 2017; 239: 115-146.
- Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018; 11: 1-10.
- Tarasiuk A, Eibl G. Nutritional Support and Probiotics as a Potential Treatment of IBD. Curr Drug Targets. 2020; 21: 1417-1427.
- Dall'Oglio VM, Balbinot RS, Muscope ALF, Castel MD, Souza TR, Macedo RS, et al. Epidemiological profile of inflammatory bowel disease in Caxias do Sul, Brazil: a cross-sectional study. Sao Paulo Med J. 2020.
- Fabisiak N, Fabisiak A, Chmielowiec-Korzeniowska A, Tymczyna L, Kamysz W, Kordek R, et al. Anti-inflammatory and antibacterial effects of human cathelicidin active fragment KR-12 in the mouse models of colitis: A novel potential therapy of inflammatory inflammatory bowel diseases. Pharmacol Rep. 2020.
- Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017; 92: 1088-1103.
- Abraham B, Quigley EMM. Antibiotics and probiotics in inflammatory bowel disease: when to use them? Frontline Gastroenterol. 2020; 11: 62-69.
- Yilmaz I, Dolar ME, Ozpinar H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk J Gastroenterol. 2019; 30: 242-253.
- Bin L, Yang F, Lu D, Lin Z. Specific immunotherapy plus Clostridium butyricum alleviates ulcerative colitis in patients with food allergy. Sci Rep. 2016; 6: 25587.
- Jarmakiewicz-Czaja S, Piatek D, Filip R. The Influence of Nutrients on Inflammatory Bowel Diseases. J Nutr Metab. 2020; 2020: 2894169.
- Goulart RA, Barbalho SM, Lima VM, Souza GA, Matias JN, Araújo AC, et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn's Disease: A Systematic Review. J Med Food. 2020.
- Jia Y, Anwaar S, Li L, Yin Z, Ye Z, Huang Z. A new target for the treatment of inflammatory bowel disease: Interleukin-37. Int Immunopharmacol. 2020; 83: 106391.
- Ahluwalia B, Moraes L, Magnusson MK, Ohman L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018; 53: 379-389.
- Goulart RA, Barbalho SM, Rubira CJ, Araújo AC, Lima VM, Rogerio Leoni B, et al. Curcumin therapy for ulcerative colitis remission: systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2020; 14: 1171-1179.
- Barbalho SM, Bosso H, Salzedas-Pescinini LM, de Alvares Goulart R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complement Ther Med. 2019; 43: 148-153.
- Mazieiro R, Frizon RR, Barbalho SM, Goulart RA. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J Med Food. 2018; 21: 1077-1085.
- Du X, Xie C, Shi L, Gao H, Yang C, Liu Q. Probiotics, prebiotics, and synbiotics supplementation in prediabetes: protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020; 99: e19708.
- Parker EA, Roy T, D'Adamo CR, Wieland LS. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition. 2018; 45: 125-134.e111.
- Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017; 96: 170-178.
- O'Connell TM. The Application of Metabolomics to Probiotic and Prebiotic Interventions in Human Clinical Studies. Metabolites. 2020; 10.
- Nobili V, Mosca A, Alterio T, Cardile S, Putignani L. Fighting Fatty Liver Diseases with Nutritional Interventions, Probiotics, Symbiotics, and Fecal Microbiota Transplantation (FMT). Adv Exp Med Biol. 2019; 1125: 85-100.
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017; 8: 172-184.
- Bringiotti R, Ierardi E, Lovero R, Losurdo G, Di Leo A, Principi M. Intestinal microbiota: The explosive mixture at the origin of inflammatory bowel disease? World J Gastrointest Pathophysiol. 2014; 5: 550-559.
- Al-Bawardy B, Shivashankar R, Proctor DD. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front Pharmacol. 2021; 12: 651415.
- Din AU, Hassan A, Zhu Y, Zhang K, Wang Y, Li T, et al. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J Nutr Biochem. 2020; 79: 108353.
- Manansala M, Baughman R, Novak R, Judson M, Sweiss N. Management of immunosuppressants in the era of coronavirus disease-2019. Curr Opin Pulm Med. 2021; 27: 176-183.
- Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N,et al. Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. J Nutr Biochem. 2018; 61: 129-139.
- Fitzgibbon G, Mills KHG. The microbiota and immune-mediated diseases: Opportunities for therapeutic intervention. Eur J Immunol. 2020; 50: 326-337.
- Cappello F, Rappa F, Canepa F, Carini F, Mazzola M, Tomasello G, et al. Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. Int J Mol Sci. 2019; 20.
- Fan H, Du J, Liu X, Zheng WW, Zhuang ZH, Wang CD, Gao R. Effects of pentasa-combined probiotics on the microflora structure and prognosis of patients with inflammatory bowel disease. Turk J Gastroenterol. 2019; 30: 680-685.
- Kamarli Altun H, Akal Yildiz E, Akin M. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk J Gastroenterol. 2019; 30: 313-320.
- Bamba S, Takahashi K, Imaeda H, Nishida A, Kawahara M, Inatomi O, et al. Effect of fermented vegetable beverage containing Pediococcus pentosaceus in patients with mild to moderate ulcerative colitis. Biomed Rep. 2018; 9: 74-80.
- Matsuoka K, Uemura Y, Kanai T, Kunisaki R, Suzuki Y, Yokoyama K, et al. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig Dis Sci. 2018; 63: 1910-1919.
- Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016; 160: 372-377.
- Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016; 28: 67-74.
- Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. 2016; 46: 939-949.
- Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13: 928-935.e922.
- Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Effects of Probiotics on Gut Microbiota in Patients with Inflammatory Bowel Disease: A Double-blind, Placebo-controlled Clinical Trial. Korean J Gastroenterol. 2015; 65: 215-221.
- Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol. 2015; 21: 5985-5994.
- Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol. 2013; 47: 437-439.