Japanese Journal of Gastroenterology Research
Research Article - Open Access, Volume 1
Evaluation of efficacy and tolerability of hepameg (probiotics + eicosapentaenoic acid + docosahexaenoic acid + vitamin E) in the management of non-alcoholic fatty liver disease
*Corresponding Author: Arif A Faruqui
Clinical Pharmacologist, Department of Pharmacology Opp. K. B. Bhabha Hospital Bandra West, Mumbai-400050 Maharashtra, India.
Email: drfaruqui@gmail.com
Received : Sep 21, 2021
Accepted : Nov 01, 2021
Published : Nov 06, 2021
Archived : www.jjgastro.com
Copyright : © Faruqui AA (2021).
Abstract
Background: Non-Alcoholic Fatty Liver Disease (NAFLD) has currently emerged as common liver disorder compared to alcoholic liver disease. Available treatments are associated with certain side-effects. Recently a relation between gut and liver, i.e. gut-liver axis has been studied extensively. Modulation of gut may provide a natural mechanism to improve NAFLD associated complications.
Aim: To evaluate the efficacy and tolerability of Hepameg. Patients were given 1 gram Hepameg sachet containing probiotics, omega-3-fatty acids and vitamin E twice a day for 12 weeks.
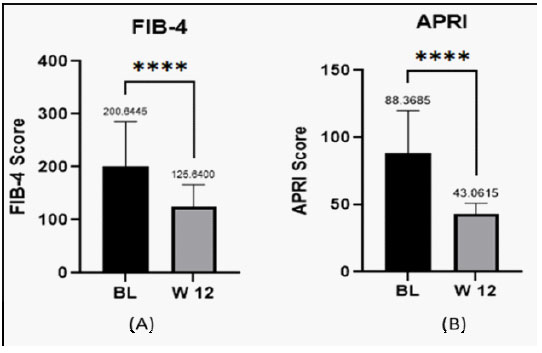
Methods: An open-label, non-comparative post marketing study was conducted in 20 subjects with NAFLD. Physical and biochemical investigations were performed and inflammatory markers were assessed at baseline and after 12 weeks. Primary outcomes were change in scores for Fibrosis-4 (FIB-4) and AST to Platelet Ratio Index (APRI) at baseline and week 12. Secondary outcomes were global assessment of efficacy by physician and patients on a pre-defined 0 to 4 score scale.
Results: After 12 weeks of Hepameg therapy, all patients reported significant improvement in FIB-4 and APRI scores. On the predefined scale of global assessment, physicians and patients reported excellent efficacy and tolerability rating for Hepameg.
Conclusion: The results of the study showed that Hepameg is clinically effective and safe in the management of NAFLD.
Keywords: non-alcoholic fatty liver disease; probiotics; omega-3-fatty acids; vitamin E.
Citation: Faruqui AA. Evaluation of efficacy and tolerability of hepameg (probiotics + eicosapentaenoic acid + docosahexaenoic acid + vitamin E) in the management of non-alcoholic fatty liver disease. Japanese J Gastroenterol Res. 2021; 1(7): 1031.
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) is a broad term used to describe a continuum of liver conditions typically ranging from steatosis (fatty liver) to non-alcoholic steatohepatitis (NASH), steatohepatitis with fibrosis, and cirrhosis. NAFLD can result in liver injury and fibrosis in the absence of other liver disease etiologies such as medications, viral hepatitis, and significant alcohol consumption. NAFLD has currently emerged as common liver disorder due to increased prevalence of obesity and weight related metabolic comorbidities [1]. Currently, the global prevalence of NAFLD is estimated to be 25%, with highest prevalence in the Middle East (32%) and South America (31%) followed by Asia (27%), United States (24%), Europe (23%), and Africa with the lowest prevalence (14%) [2]. NAFLD is increasing in prevalence, associated with a significant number of comorbidities and is rapidly becoming the most common reason for liver transplant. Therefore, patients with NAFLD should begin treatment as soon as the diagnosis is made [2,3]. Although over 25 medications are undergoing phase 2 or 3 clinical trials, currently there are no specific medications that directly treat NAFLD [4]. The interactions between the liver and the gut, the so-called "gut-liver axis" plays an important role in NAFLD onset and progression. Compelling evidences are available that link gut microbiome, intestinal barrier integrity, and NAFLD. Therapeutic manipulations with probiotics are potential agents for NAFLD management by modulating gut microbiota and maintaining intestinal barrier integrity [5]. NAFLD is generally associated with elevated n-6 and a deficiency of n-3 polyunsaturated fatty acids (PUFAs) in the diet. Studies have demonstrated that lower PUFA content and a higher n-6/n-3 ratio in NAFLD patients [6]. Meta-analyses and clinical trials have evaluated dietary C20–22 omega-3 PUFA supplementation to improve health outcomes in adults and children with pre-existing NAFLD. Diets supplemented with docosahexaenoic acid (DHA) alone or in combination with eicosapentaenoic acid (EPA) significantly reduces liver fat in NAFLD patients [7]. It is known that vitamin E is the major lipid-soluble chain-breaking antioxidant found in the stores of human body. Although NAFLD pathogenesis and its progression to fibrosis remains to be understood, it is believed that oxidative stress plays a major part in producing the lethal hepatocyte injury. Vitamin E in addition to its anti-oxidative properties, exert anti-atherogenic and anti-inflammatory activities. Therefore, by targeting oxidative stress components, vitamin E may prove to be an effective therapeutic options in patients with NASH. Also, European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines consider vitamin E as a potential short-term treatment for non-diabetic adults with biopsy-proven NASH [8]. In the light of above findings, the current study evaluated the efficacy and tolerability of Hepameg (combination of probiotics, omega-3 fatty acids and vitamin E) in the management of NAFLD.
Materials and methods
Study design and patients
This study was an open, non-comparative, post marketing study with 20 patients (age >18 years) conducted in a clinic in India. Patients suffering from liver dysfunction presenting with fatty liver confirmed with physical and biochemical investigations with elevated inflammatory marker were recruited.
Treatment and duration of treatment
Patients were given 1 gram Hepameg sachet containing probiotics, omega-3-fatty acids and vitamin E twice a day for 12 weeks.
Investigations
Physical and biochemical investigations, primary and secondary endpoints were evaluated at baseline and after treatment with Hepameg for 12-weeks. Physical investigations involved body mass index (BMI), waist circumference (WC) and blood pressure (BP). Biochemical investigations included platelet count, fasting glucose, serum glutamic oxaloacetic transaminase/ aspartate aminotransferase (SGOT/AST), serum glutamic pyruvic transaminase/alanine aminotransferase (SGPT/ALT), gamma-glutamyl transferase (GGT), total bilirubin, total cholesterol, triglycerides, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) and very low density lipoprotein (VLDL). Inflammatory marker evaluated was hs-CRP. Physical and biochemical values along with inflammatory markers were assessed and compared at baseline and after 12 weeks.
Primary and secondary outcomes
Primary outcomes were change in scores for Fibrosis-4 (FIB-4) and AST to Platelet Ratio Index (APRI) at baseline and week 12. Secondary outcomes included global assessment of efficacy by physician and patients on a pre-defined 0 to 4 score scale.
Adverse events
Adverse event was recorded on a scale of scores 1 to 3 (1=mild, 2=moderate, 3=severe). Outcome of action taken on adverse events were recorded on a scale of scores 1 to 5 (1=unchanged, 2=improved, 3=resolved, 4=worsened, 5=death). Global assessment of efficacy and tolerability by physician and patients respectively was assessed on a pre-defined five point scale of scores 0 to 4 (1=none, 2=poor, 3=good, 4=excellent).
Statistical analysis
Statistical analysis was done by "paired t-test" for each parameters compared with change from baseline to 12-weeks. The minimum level of significance was fixed at 95% confidence limit and P <0.05 was considered as significant. All the bio-statistical analysis was performed by using Graph Pad Prism 9 version 9.2.0.
Results
All included 20 patients completed the study at the end of 12 weeks. Out of 20 patients 12 were males 8 were females. Average age was 46.15 years.
Physical investigations
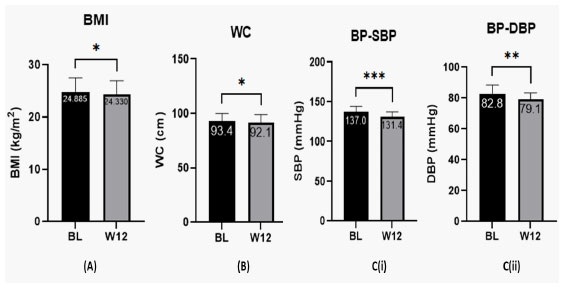
Patients were evaluated for BMI, WC and BP at baseline and week 12 after treatment with Hepameg. A significant improvement in BMI (P = 0.0152), WC (P = 0.0249), and BP (SBP: P = 0.0004; DBP: P = 0.0047) was observed from baseline after treatment with Hepameg for 12 weeks (Figure 1).
Biochemical investigations
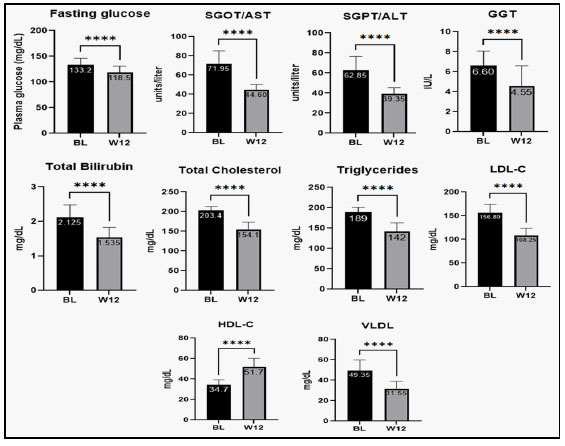
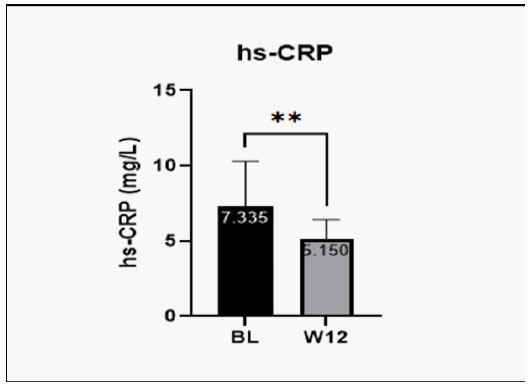
Patients were investigated for change from baseline in biochemical parameters such as, platelet count, fasting glucose, SGOT/AST, SGPT/ALT, GGT, total bilirubin, total cholesterol, triglycerides, LDL-C, HDL-C and VLDL after treatment with Hepameg for 12-weeks. At week 12, change in platelet count was not statistically significant. Other parameters such as, fasting glucose, SGOT/AST, SGPT/ALT, GGT, total bilirubin, total cholesterol, triglycerides, LDL-C, and VLDL reduced significantly at week-12 after treatment with Hepameg (Figure 2). HDL-C was significantly improved from the baseline after Hepameg treatment as reported at week-12 (Figure 2(I)). Major inflammatory biomarker hs-CRP was also noted to reduce significantly from baseline after treatment with Hepameg at week-12 (Figure 3).
SGOT/AST: Serum glutamic oxaloacetic transaminase/ aspartate aminotransferase; SGPT/ALT: Serum glutamic pyruvic transaminase/alanine aminotransferase; GGT: gamma-glutamyl transferase
LDL-C: Low density lipoprotein cholesterol, HDL-C: High density lipoprotein cholesterol; VLDL: Very low density lipoprotein. ****P <0.0001.
Global assessment of efficacy and tolerability
Efficacy and tolerability assessed on Global assessment score was 4 (excellent) by all the 20 patients. Physicians also reported Global assessment score of 4 suggesting excellent tolerability and efficacy of Hepameg.
Adverse events
No complaints of adverse events were reported by the patients during and after completion of clinical trial at week- 12 with Hepameg treatment.
Discussion
In this open, non-comparative, post marketing study, Hepameg (probiotics in combination with omeg-3 fatty acid and vitamin E) was reported to significantly reduce BMI, WC, and BP in patients with NAFLD. The combination was also reported to significantly reduce inflammatory parameters such as hs-CRP. Significant improvement in fasting glucose, SGOT/AST, SGPT/ALT, GGT, Total bilirubin, Total Cholesterol, Triglycerides, LDL-C, HDL-C, VLDL was also reported on treatment with Hepameg. FIB-4 score (used to measure fibrosis) and APRI scores (measure health of liver) were also significantly reduced with Hepameg. Hepameg was reported to have excellent safety and tolerability profile. This suggests the use of probiotics along with omega-3 fatty acids and vitamin E shows excellent efficacy in reducing liver fibrosis and associated parameters in patients with NAFLD.
The combined effect of probiotics and omega-3 PUFAs have already been studied in two clinical trials. The findings of our study are consistent with the findings of these studies. The combination of probiotics with PUFAs was reported to significantly reduce lipid parameters and chronic inflammatory markers; and improved insulin sensitivity and fatty liver index (FLI). Study conducted by Rajkumar et al. noted that probiotic supplementation results in significant reductions of total cholesterol, triglyceride, LDL, and VLDL and increased HDL-C levels (p <0.05). This study also reported reduction of inflammatory marker hsCRP as observed in our study [9]. Another double-blind single centre randomized placebo-controlled trial conducted by Kobyliak N, et al. showed that probiotics with omega-3 significantly reduced FLI from baseline and also resulted in decreased serum gamma-glutamyl transpeptidase, triglycerides, and total cholesterol levels in type-2 diabetic patients with NAFLD [10].
A systematic review and meta-analysis of 22 clinical studies with probiotics supplementation in NAFLD patients showed decrease in weight, body mass index, improved liver function (reduced ALT and AST), improved lipid profile which was consistent with our study. In our study the liver function was significantly improved with reduction of ALT and AST [11].
Probiotics are known to restore intestinal barrier integrity and reduce the inflammatory mediators contributing to NAFLD [12]. The modulation of gut microbiota by probiotic results in changed bile acid absorption, down regulation of FGR-FGF15 (FGF-19 in humans) axis, and increased BA neosynthesis in hepatocytes thus, enhancing the BA deconjugation and fecal excretion in mice [13]. This may contribute to reduced inflammatory markers and improved liver function in our study. n-3 PUFA supplements effectively reduces TG levels resulting in better glycemic control, as shown in a study of NASH patients with diabetes. n-3 PUFAs activates the peroxisome proliferator-activated receptor (PPAR) alpha (stimulates fatty acid oxidation) and PPAR gamma (increases insulin sensitivity). n-3 PUFAs inhibits hepatic lipogenesis, and reduces hepatic reactive oxygen species. NAFLD patients are reported to have a greater deficiency of n-3 PUFAs in the diet than healthy controls and a greater n-6/n-3 ratio in NAFLD patients increased lipogenesis resulting in steatosis [14].
Oxidative stress plays a central role in non-alcoholic steatohepatitis (NASH). Vitamin E, a lipid soluble vitamin is known to be an excellent antioxidant. Vitamin E has been used in multiple clinical trials for the treatment of NAFLD or NASH, which reported improvement in liver biochemistries and histology [15]. Recent two large trials, Pioglitazone or Vitamin E for NASH Study (PIVENS) and Treatment of nonalcoholic fatty liver disease in children (TONIC) showed that Vitamin E improves hepatocellular ballooning and NASH; suggesting reduced risk of disease progression and cytoskeletal injury in both adults and children [16].
Thus, based on available clinical studies and current clinical study probiotics in combination with n-3 PUFAS, and Vitamin E via various modes of action may synergistically reduce the NAFLD progression.
Conclusion
Hepameg (combination of probiotics, omega-3 fatty acids and vitamin E) is safe and effective option for the management of NAFLD. Hepameg was well-tolerated with significant improvement in liver functions of patients with NAFLD.
Declarations
Acknowledgements: Author acknowledges the contribution of Ms. Richa Deliwala, M.Pharm (Pharmacology) and Mr. Rafique Sheikh for their contribution in doing literature search & helping in the preparation of manuscript.
References
- Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh, et al. Evolution of NAFLD and Its Management. Nutrition in clinical practice: Official publication of the American Society for Parenteral and Enteral Nutrition. 2020; 35: 72–84.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md.). 2016; 64: 73–84.
- Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology (Baltimore, Md.). 2016; 64: 1577–1586.
- Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nature medicine. 2018; 24: 908–922.
- Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic efatty liver disease. Clinical biochemistry. 2015; 48: 923–930.
- Lu W, Li S, Li J, Wang J, Zhang R, et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterology research and practice. 2016; 1459790.
- Spooner MH, Jump DB. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: where do we stand?. Current opinion in clinical nutrition and metabolic care. 2019; 22: 103–110.
- El Hadi H, Vettor R, Rossato M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth?. Antioxidants (Basel, Switzerland). 2018; 7: 12.
- Rajkumar H, Mahmood N, Kumar M, Varikuti SR, et al. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators of inflammation. 2014; 2014; 348959.
- Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto et al. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva medica. 2018; 109: 418–428.
- Tang Y, Huang J, Zhang WY, Qin S, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Therapeutic advances in gastroenterology. 2019; 12: 1756284819878046.
- Meroni M, Longo M, Dongiovanni P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients. 2019; 11: 2642.
- Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell reports. 7: 12–18.
- Lu W, Li S, Li J, Wang J, et al. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterology research and practice. 2016; 1459790.
- Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Current opinion in clinical nutrition and metabolic care. 2012; 15: 641–648.
- Beaton MD. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Canadian journal of gastroenterology = Journal canadien de gastroenterology. 2012; 26: 353–357.